Citrusin BCAS# 105279-10-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 105279-10-5 | SDF | Download SDF |
| PubChem ID | 11972318 | Appearance | Powder |
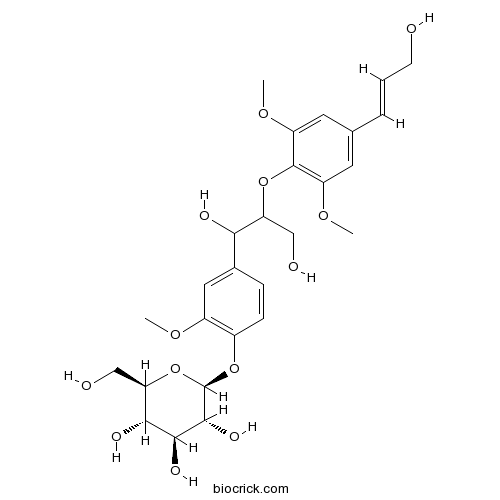
| Formula | C27H36O13 | M.Wt | 568.6 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3R,4S,5S,6R)-2-[4-[1,3-dihydroxy-2-[4-[(E)-3-hydroxyprop-1-enyl]-2,6-dimethoxyphenoxy]propyl]-2-methoxyphenoxy]-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | COC1=CC(=CC(=C1OC(CO)C(C2=CC(=C(C=C2)OC3C(C(C(C(O3)CO)O)O)O)OC)O)OC)C=CCO | ||
| Standard InChIKey | XMGKCJUCYBLMBY-ONCYFVLKSA-N | ||
| Standard InChI | InChI=1S/C27H36O13/c1-35-17-11-15(6-7-16(17)39-27-25(34)24(33)23(32)21(13-30)40-27)22(31)20(12-29)38-26-18(36-2)9-14(5-4-8-28)10-19(26)37-3/h4-7,9-11,20-25,27-34H,8,12-13H2,1-3H3/b5-4+/t20?,21-,22?,23-,24+,25-,27-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Citrusin B exhibits moderate in vitro inhibitory effect on tobacco mosaic virus replication with IC₅₀ values 0.26 mmol L⁻1. |
| In vitro | A new seco-neolignan glycoside from the root bark of Ailanthus altissima.[Pubmed: 21916771 ]Nat Prod Res. 2012;26(15):1375-80.
|
| Structure Identification | Zhongguo Zhong Yao Za Zhi. 2013 Jul;38(13):2118-24.A new lignan from stems of Sargentodoxa cuneata.[Pubmed: 24079238]Sargentodoxae Caulis was prepared from the stems of Sargentodoxa cuneata. |

Citrusin B Dilution Calculator

Citrusin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7587 mL | 8.7935 mL | 17.5871 mL | 35.1741 mL | 43.9676 mL |
| 5 mM | 0.3517 mL | 1.7587 mL | 3.5174 mL | 7.0348 mL | 8.7935 mL |
| 10 mM | 0.1759 mL | 0.8794 mL | 1.7587 mL | 3.5174 mL | 4.3968 mL |
| 50 mM | 0.0352 mL | 0.1759 mL | 0.3517 mL | 0.7035 mL | 0.8794 mL |
| 100 mM | 0.0176 mL | 0.0879 mL | 0.1759 mL | 0.3517 mL | 0.4397 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Genistein 7-O-beta-D-glucopyranoside-4'-O-[alpha-L-rhamnopyranosyl-(1->2)-beta-D-glucopyranoside]
Catalog No.:BCN8772
CAS No.:70404-42-1
- 1,7-Diphenyl-5-hydroxy-4,6-hepten-3-one
Catalog No.:BCN8771
CAS No.:87095-77-0
- Pueroside C
Catalog No.:BCN8770
CAS No.:112343-16-5
- Momordicoside X
Catalog No.:BCN8769
CAS No.:1333321-50-8
- Pueroside B
Catalog No.:BCN8768
CAS No.:100692-54-4
- Formononetin-8-C-beta-D-apiofuranosyl-(1->6)-O-beta-D-glucopyranoside
Catalog No.:BCN8767
CAS No.:1147858-78-3
- Daidzein-4'-glucoside
Catalog No.:BCN8766
CAS No.:58970-69-7
- Rhamnocitrin 3-apiosyl-(1→2)-glucoside
Catalog No.:BCN8765
CAS No.:148031-68-9
- Orientalide
Catalog No.:BCN8764
CAS No.:72704-05-3
- 3,7,23,24-tetrahydroxycucurbita-5,25-dien-19-al
Catalog No.:BCN8763
CAS No.:1446447-97-7
- (1,5E,11E)-tridecatriene-7,9-diyne-3,4-diacetate
Catalog No.:BCN8762
CAS No.:201012-14-8
- Rhamnocitrin 3-glucoside
Catalog No.:BCN8761
CAS No.:41545-37-3
- Licoricesaponin H2(18beta,20alpha-Glycyrrhizic acid)
Catalog No.:BCN8774
CAS No.:118441-85-3
- 3-O-[5'''-O-feruloyl-beta-D-apiofuranosyl(1'''->2'')-beta-D-glucopyranosyl] rhamnocitrin
Catalog No.:BCN8775
CAS No.:148210-00-8
- Yuanhunine
Catalog No.:BCN8776
CAS No.:104387-15-7
- Silychristin B
Catalog No.:BCN8777
CAS No.:879325-58-3
- Maculosidin
Catalog No.:BCN8778
CAS No.:522-19-0
- Isonardosinone
Catalog No.:BCN8779
CAS No.:27062-01-7
- Dehydrojuncusol
Catalog No.:BCN8780
CAS No.:117824-04-1
- 7-(4-hydroxy-3-methoxyphenyl)-1-phenylhept-4-en-3-one (DPHB)
Catalog No.:BCN8782
CAS No.:79559-60-7
- Specioside B
Catalog No.:BCN8784
CAS No.:126589-95-5
- Lanceolarin
Catalog No.:BCN8785
CAS No.:15914-68-8
- 5-Hydroxy-1-(4-hydroxyphenyl)-7-phenyl-3-heptanone (AO 2210)
Catalog No.:BCN8786
CAS No.:105955-04-2
- 1-Phenyl-2-propanol
Catalog No.:BCN8787
CAS No.:14898-87-4
[Chemical constituents from whole plants of Aconitum tanguticum].[Pubmed:24380304]
Zhongguo Zhong Yao Za Zhi. 2013 Sep;38(17):2818-25.
Nineteen compounds were isolated from the whole plants of Aconitum tanguticum by means of various of chromatographic techniques such as silica gel, ODS, sephadex LH-20 and preparative HPLC, and their structures were elucidated as syringin (1), vanillic acid-4-O-beta-D-allopyranoside (2), (E) -ferulic acid 4-O-beta-D-allopyranoside (3), (E) -ferulic acid-4-O-beta-glucopysoside (4), (E) -sinapic acid-4-O-beta-glucopyranoside (5), (E) 4-hydroxycinnamyl alcohol 4-O-beta-D-glucopyranoside (6), quercetin 3-O-alpha-L-rhamnopyranosyl-(1 --> 2) -[alpha-L-rhamnopyranosyl-(1 --> 6)] -beta-D-galactopyranoside-7-O-alpha-L-rhamnopyranoside (7), kaempferol 3-O-alpha-L-rhamnopyranosyl-(1 --> 2) - [alpha-L-rhamnopyranosyl-(1 --> 6)] -beta-D-galactopyranside-7-O-alpha-L-rhamnopyranoside (8), quercetin 3-O-alpha-L-rhamnopyranosyl-(1 --> 6) -beta-D-glucopyranoside-7-O-alpha-L-rhamnopyranoside (9), kaempferol 3-O-[beta-D-glucopyranosyl-(1 --> 3)-(4-O-trans-p-coumaroyl) ] -alpha-L-rhamnopyranosyl-(1 --> 6) -beta-D-galactopyranside-7-O-alpha-L-rhamnopyranoside (10), quercetin 3-O- [beta-D-glucopyranosyl-(1 --> 3 ) -(4-O-trans-p-coumaroyl)] -alpha-L-rhamnopyranosyl-(1--> 6) -beta-D-galactopyranoside-7-O-alpha-L-rhamnopyranoside (11), salidroside (12), 2-(3,4-dihydroxyphenyl) ethanol 1-O-beta-D-glucopyranoside (13), (7S, 8R) -dehydrodiconiferyl alcohol-9'-O-beta-D-glucopyranoside (14), Citrusin B (15), heteratisine (16), tanaconitine (17), shanzhiside methyl ester (18) and icariside B1 (19). Except compounds 4, 13, 16 and 17, the other compounds were separated from the species for the first time.
[A new lignan from stems of Sargentodoxa cuneata].[Pubmed:24079238]
Zhongguo Zhong Yao Za Zhi. 2013 Jul;38(13):2118-24.
Sargentodoxae Caulis was prepared from the stems of Sargentodoxa cuneata. Twenty compounds from the the stems of S. cuneata collected in Huangshan Mountain, Anhui province, were isolated and purified by column chromatography on macroporous resin (HPD100), silica gel, Sephadex LH-20 and semi-preparative HPLC. Their structures were elucidated on the basis of physico-chemical properties and spectral data analyses as (7R,8S)-3,3 '-5-trimethoxy-4,9-dihydroxy-4',7-expoxy-5',8-lignan-7'-en-9'-oic acid 4-O-beta-D-glucopyranoside(1), 1-O-(vanillic acid) -6-O-vanilloyl-beta-D-glucopyranoside(2), 4-hydroxyphenylethyl-6-O-coumaroyl-beta-D-glucopyranoside(3), Citrusin B(4), cinnamoside(5), (-) -isolariciresinol 4'-O-beta-D-glucopyranoside (6), (-) -isolariciresinol 4-O-beta-D-glucopyranoside (7), 1-O-(vanillic acid) -6-(3", 5"-dimethoxy-galloyl) -beta-D-glucopyranoside (8), 4-hydroxyphenyl-ethyl-6-O-(E) -caffeoyl-beta-D-glucopyranoside (9), (-)-syringaresinol 4'-O-beta-D-glucopyranoside (10), (-)-syringaresinol di-O-beta-D-glucopyranoside (11), aegineoside (12), calceolarioside B (13), 4-hydroxy-3-methoxy-acetophenone-4-O-alpha-L-rhamnopyranosyl-(1 --> 6)-beta-D-glucopyranoside (14), 4-hydroxy-3-methoxy-acetophenone-4-O-beta-D-apiofuranosyl-(1 --> 6) -beta-D-glucopyranoside (15), (-) -epicatechin (16), salidroside (17), 3,4-dihydroxy-phenyl ethyl-beta-D-glucopyranoside (18), chlorogenic acid (19) and protocatechuic acid (20). Compound 1 was a new compound and compounds 2-7 were isolated from this plant for the first time.
[One new lignan glycoside from whole plants of Senecio chrysanthemoides].[Pubmed:22032139]
Zhongguo Zhong Yao Za Zhi. 2011 Jul;36(13):1755-62.
OBJECTIVE: To investigate the chemical constituents from the whole plants of Senecio chrysanthemoides. METHOD: Constituents were isolated by using a combination of various chromatographic techniques including column chromatography over silica gel, Sephadex LH-20, and ODS C18, as well as reversed-phase HPLC. Structures of the isolates were identified by spectroscopic and chemical methods. RESULT: Eighteen glycosides were obtained from a H2O-soluble portion of an ethanolic extract of the whole plants of Senecio chrysanthemoides and their structures were elucidated as 5'-methoxyligusinenoside B (1), hyuganoside III b (2), citrusin A (3), alaschanioside A (4), Citrusin B (5), dehydrodieoniferyl alcohol 4, gamma'-di-O-beta-D-glucopyranoside (6), osmanthuside G (7), syringin (8), dehydrosyringin (9), 2-(4-hydroxy-3,5-dimethoxyphenyl) ethanol 4-O-beta-D-glucopyranoside (10), 2-phenylethyl beta-gentiobioside (11), phenethyl beta-D-glucopyranoside (12), nikoenoside (13), benzyl beta-D-glucopyranoyl (1 --> 6 ) -beta-D-glucopyranoside (14), 3,5-dimethoxy-4-hydroxybenzyl alcohol 4-O-beta-D-glucopyranoside (15), icariside B2 (16), sonchuionoside C (17), and 1-[(beta-D-glucopyranosyloxy) methyl] -5,6-dihydropyrrolizin-7-one (18). CONCLUSION: Compound 1 was a new lignan glycoside, and the remaining compounds were obtained from this plant for the first time.
A new seco-neolignan glycoside from the root bark of Ailanthus altissima.[Pubmed:21916771]
Nat Prod Res. 2012;26(15):1375-80.
A new seco-neolignan glycoside, seco-dehydrodiconiferyl alcohol-4-O-beta-D-glucopyranoside, together with eight known compounds, were obtained from the EtOH extract of the root bark of Ailanthus altissima. Their structures were elucidated based on the spectroscopic data. Three neolignan glycosides including 7,9,9'-trihydroxy-3,3',5'-trimethoxy-8-O-4'-neolignan-4-O-beta-D-glucopyranoside, sonchifolignan B and Citrusin B exhibited moderate in vitro inhibitory effect on tobacco mosaic virus replication with IC(5)(0) values 0.30, 0.35 and 0.26 mmol L(-)(1), respectively.
[Studies on phenylpropanoids from herbs of Eriophyton wallichii].[Pubmed:19216160]
Zhongguo Zhong Yao Za Zhi. 2008 Nov;33(22):2636-9.
OBJECTIVE: To study the chemical constituents of Eriophyton wallichii. METHOD: Compounds were separated and purified by column chromatographic methods, and their structures were elucidated by spectroscopic methods. RESULT: Eight phenylpropanoids were isolated and identified as martynoside (1), leucosceptoside A (2), Citrusin B (3), (+)-dehydrodiconiferyl alcohol-4, 9-beta-D-glucopyranoside (4), liriodendrin (5), velutinoside 11[ (6), jionoside B, (7), stachysoside D (8), respectively. CONCLUSION: The eight compounds were firstly isolated from E. wallichii.


