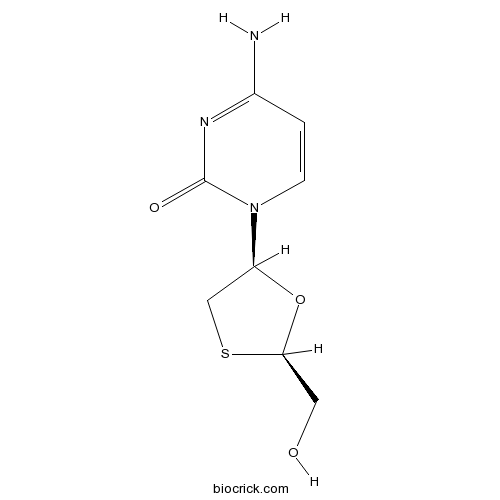
LamivudineNucleoside analog reverse transcriptase inhibitor CAS# 134678-17-4 |
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Tenofovir Disoproxil Fumarate
Catalog No.:BCC1108
CAS No.:202138-50-9
- Entecavir Hydrate
Catalog No.:BCC1109
CAS No.:209216-23-9
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
- Didanosine
Catalog No.:BCC3763
CAS No.:69655-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 134678-17-4 | SDF | Download SDF |
| PubChem ID | 60825 | Appearance | Powder |
| Formula | C8H11N3O3S | M.Wt | 229.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (218.09 mM; Need ultrasonic) H2O : ≥ 50 mg/mL (218.09 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one | ||
| SMILES | C1C(OC(S1)CO)N2C=CC(=NC2=O)N | ||
| Standard InChIKey | JTEGQNOMFQHVDC-NKWVEPMBSA-N | ||
| Standard InChI | InChI=1S/C8H11N3O3S/c9-5-1-2-11(8(13)10-5)6-4-15-7(3-12)14-6/h1-2,6-7,12H,3-4H2,(H2,9,10,13)/t6-,7+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Lamivudine is a potent nucleoside analog reverse transcriptase inhibitor with an IC50 of 2.7 mM.
Target: NRTIs; HIV
Lamivudine is a reverse transcriptase inhibitor and zalcitabine analog in which a sulfur atom replaces the 3' carbon of the pentose ring. It is used to treat Human Immunodeficiency Virus Type 1 (HIV-1) and hepatitis B (HBV). Lamivudine can inhibit virus replication rapidly and improveliver function, prevent exacerbation in patients with advanced schistosomiasis with chronic B hepatitis [1]. IC50 value of lamivudine on B. adolescentis was 200 microg x mL(-1), and the IC50 values of lamivudine on S. dysenteriae and E. coli were higher than 3 000 microg x mL(-1) and 6 000 microg x mL(1), respectively. Therefore, lamivudine made different inhibitory effects on the three bacteria, in which the B. adolescentis was most susceptible to lamivudine. This work showed that taking lamivudine chronically is likely to affect the balance of good flora in the intestinal tract, and might increase endotoxin release, leading to inflammation and disease progression in hepatopathy [2]. References: | |||||

Lamivudine Dilution Calculator

Lamivudine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3619 mL | 21.8093 mL | 43.6186 mL | 87.2372 mL | 109.0465 mL |
| 5 mM | 0.8724 mL | 4.3619 mL | 8.7237 mL | 17.4474 mL | 21.8093 mL |
| 10 mM | 0.4362 mL | 2.1809 mL | 4.3619 mL | 8.7237 mL | 10.9046 mL |
| 50 mM | 0.0872 mL | 0.4362 mL | 0.8724 mL | 1.7447 mL | 2.1809 mL |
| 100 mM | 0.0436 mL | 0.2181 mL | 0.4362 mL | 0.8724 mL | 1.0905 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A potent nucleoside analog reverse transcriptase inhibitor (nRTI). An analog of cytidine that can inhibit both types (1 and 2) of HIV reverse transcriptase as well as the reverse transcriptase of hepatitis B. Needs to be phosphorylated to its triphosphate form before it is active.
- GSK343
Catalog No.:BCC1607
CAS No.:1346704-33-3
- GSK621
Catalog No.:BCC6517
CAS No.:1346607-05-3
- GSK126
Catalog No.:BCC1604
CAS No.:1346574-57-9
- GSK503
Catalog No.:BCC6386
CAS No.:1346572-63-1
- ML 240
Catalog No.:BCC5604
CAS No.:1346527-98-7
- Zinc Pyrithione
Catalog No.:BCC5008
CAS No.:13463-41-7
- Eriocitrin
Catalog No.:BCN1208
CAS No.:13463-28-0
- Stavudine sodium
Catalog No.:BCC4263
CAS No.:134624-73-0
- ML 218 hydrochloride
Catalog No.:BCC6207
CAS No.:1346233-68-8
- SA 57
Catalog No.:BCC6280
CAS No.:1346169-63-8
- Planchol E
Catalog No.:BCN6882
CAS No.:1346137-02-7
- LY2795050
Catalog No.:BCC1719
CAS No.:1346133-08-1
- Linderane
Catalog No.:BCN5023
CAS No.:13476-25-0
- A 412997 dihydrochloride
Catalog No.:BCC6224
CAS No.:1347744-96-0
- 6''-O-Acetylglycitin
Catalog No.:BCN3866
CAS No.:134859-96-4
- 8-M-PDOT
Catalog No.:BCC6901
CAS No.:134865-70-6
- 4-P-PDOT
Catalog No.:BCC6900
CAS No.:134865-74-0
- SAR245409
Catalog No.:BCC2534
CAS No.:934493-76-2
- Taccalonolide AJ
Catalog No.:BCN2971
CAS No.:1349904-82-0
- 2,2'-Dithiobisbenzanilide
Catalog No.:BCC8497
CAS No.:135-57-9
- Pseudotropine
Catalog No.:BCN1932
CAS No.:135-97-7
- Z-Hyp-OH
Catalog No.:BCC3257
CAS No.:13504-85-3
- Clopidogrel bisulfate
Catalog No.:BCC8917
CAS No.:135046-48-9
- MK-5172 hydrate
Catalog No.:BCC1763
CAS No.:1350462-55-3
Evolutionary trends of resistance mutational patterns of HBV reverse transcriptase over years (2002-2012) of different treatment regimens: The legacy of lamivudine/adefovir combination treatment.[Pubmed:28322924]
Antiviral Res. 2017 Jul;143:62-68.
Antiviral therapy has revolutionized treatment of chronic HBV infections. First generation compounds, Lamivudine and adefovir, displayed a high rate of treatment failures, and have been replaced by more potent compounds with high genetic barrier to resistance. However, the evolution of the virus towards resistance due the use of first generation compounds may still provide useful information for a better management of current antivirals. A single center sequence database including 705 HBV reverse transcriptase sequences from patients failing antiviral treatments (2002-2012) has been statistically analyzed to highlight viral evolution in relationship to the use of antiviral compounds and to their associations/sequencing in those years. The influence of viral genotypes and polymorphisms on resistance-related mutational patterns was also investigated. This study documents how, after the first years of antiviral therapy, the use of adefovir as an add-on strategy allowed a consistent reduction treatment failures. It also documents the effects of the initial misuse of entecavir in Lamivudine experienced patients. In the latest years, the correct use of entecavir and the introduction of tenofovir allowed further curbing of resistance-related treatment failures, which virtually disappeared. Furthermore, the study allows a better understanding of how viral genotype (A vs D) conditions specific mutational pathways to resistance against Lamivudine and entecavir, and demonstrates that the use of adefovir in Lamivudine experienced patients is associated to peculiar mutational patterns, in particular A181V + F/Y221L. Despite some concern may arise for patients previously treated with Lamivudine/adefovir, in sequence or combination, where the virus may have developed a lower genetic barrier against resistance to tenofovir, the outlook of antiviral treatment of HBV infection should be quite optimistic.
Evolution of blood-associated HIV-1 DNA levels after 48 weeks of switching to atazanavir/ritonavir+lamivudine dual therapy versus continuing triple therapy in the randomized AtLaS-M trial.[Pubmed:28333353]
J Antimicrob Chemother. 2017 Jul 1;72(7):2055-2059.
Objectives: The AtLaS-M randomized trial showed that in patients with HIV-1 RNA <50 copies/mL on atazanavir/ritonavir + two NRTIs, switching to a dual therapy with atazanavir/ritonavir+Lamivudine had superior efficacy as compared with continuing the previous triple therapy. This substudy was designed to evaluate at 48 weeks the impact of the dual therapy versus the three-drug atazanavir/ritonavir-based therapy on the HIV-1 cellular reservoir as reflected by the quantification of blood-associated HIV-1 DNA levels. Methods: In a representative subset of 201 of 266 randomized patients (104 in the dual-therapy arm and 97 in the triple-therapy arm) total HIV-1 DNA levels in whole blood at baseline and after 48 weeks and factors associated with the HIV-1 DNA levels were evaluated. Results: The mean baseline HIV-1 DNA levels (2.47 log 10 copies/10 6 leucocytes) were comparable between arms. A significant mean decrease between baseline and week 48 was observed: -0.069 log 10 copies/10 6 leucocytes in the dual-therapy arm ( P = 0.046) and -0.078 in the triple-therapy arm ( P = 0.011); the mean difference between arms was -0.009 ( P = 0.842). Nadir CD4 count was inversely correlated with baseline HIV-1 DNA ( P = 0.009); longer duration of ART and lower nadir CD4 correlated with a less prominent HIV-1 DNA decrease (both P < 0.005). Higher baseline HIV-1 DNA was associated with residual viraemia at week 48 ( P = 0.031). Conclusions: When compared with continuing three-drug therapy, atazanavir/ritonavir+Lamivudine dual therapy resulted in a similar decline in HIV-1 DNA levels in patients with sustained virological suppression. These data support the safety of this simplified treatment strategy in terms of its effect on the cellular HIV-1 reservoir.
[Effect of lamivudine and silymarin on liver fibrosis-relevant factors in HBV transgenic mice with alcohol drinking].[Pubmed:28364097]
Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017 Mar 28;42(3):257-263.
OBJECTIVE: To observe the role of lamividine and silymarin preventing and curing liver fibrosis-relevant factors induced by alcohol drinking in hepatitis B virus (HBV) transgenic mice (Tg mice). Methods: Forty HBV-Tg BALB/C mice with 1.3 copy were randomly divided into 4 groups: a control group, a model group, a Lamivudine group and a silymarin group. Tg mice in control group were treated with normal saline via intragastric administration; Tg-mice in the model group were treated with 50% alcohol (5 mL/kg) once a day via intragastric administration; while Tg-mice in Lamivudine group and silymarin group were treated with alcohol (5 mL/kg) plus laminvudine (100 mg/kg) and silymarin (200 mg/kg) once a day via intragastric administration respectively. All groups were raised for 10 weeks. The levels of HBV-DNA copy number, ALT, AST in serum, the degree of inflammation, the degree of fibrosis, the mRNA expression levels of TGF-beta1, Smad3, Smad7 and connective tissue growth factor (CTGF), and the protein expression levels of TGF-beta1, CTGF and alpha-SMA in liver tissue were detected. All the images were scanned with electronic computer and the data were analyzed with SPSS13.0 software. Results: Compared with the control group, liver injury were significantly aggravated, while HBV-DNA copies, mRNA levels of TGF-beta1, Smad3, Smad7 and CTGF as well as the protein levels of TGF-beta1, CTGF and alpha-SMA were significantly increased (P<0.05). Compared with the model group, liver injury were significantly attenuated in silymarine group and Lamivudine group, while mRNA levels of TGF-beta1, Smad3 and CTGF as well as the protein levels of TGF-beta1, CTGF and alpha-SMA were significantly decreased; mRNA level of Smad7 was further increased (P<0.05); the levels of ALT and AST in serum were decreased in the silymarine group (P<0.05). Conclusion: Lamivudine and silymarin relieve the histological damage in the liver of alcohol-fed Tg mice. The mechanisms for the beneficial effects of Lamivudine or silymarin might be related to inhibiting the expression of TGF-beta1, Smad3 and CTGF, modulating the expression of Smads and suppressing the activation of HSC.
Semi-continuous multi-step synthesis of lamivudine.[Pubmed:28362445]
Org Biomol Chem. 2017 Apr 18;15(16):3444-3454.
We report the first continuous flow synthesis of Lamivudine, an antiretroviral drug used in the treatment of HIV/AIDS and hepatitis B. The key intermediate (5-acetoxy oxathiolane) was prepared by an integrated two step continuous flow process from l-menthyl glyoxalate hydrate in a single solvent, in 95% overall conversion. For the crucial glycosidation reaction, using pyridinium triflate as the novel catalyst, an improved conversion of 95% was obtained. The overall isolated yield of the desired isomer of Lamivudine (40%) was improved in the flow synthesis compared to the batch process.


