8-M-PDOTMelatonin agonist CAS# 134865-70-6 |
- PI 828
Catalog No.:BCC7494
CAS No.:942289-87-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 134865-70-6 | SDF | Download SDF |
| PubChem ID | 6604799 | Appearance | Powder |
| Formula | C14H19NO2 | M.Wt | 233.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in ethanol and to 100 mM in DMSO | ||
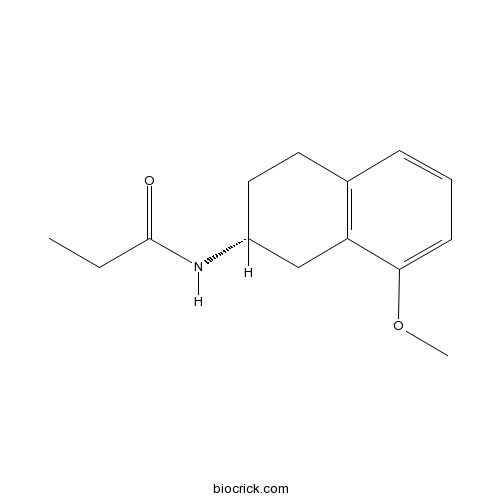
| Chemical Name | N-[(2R)-8-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl]propanamide | ||
| SMILES | CCC(=O)NC1CCC2=C(C1)C(=CC=C2)OC | ||
| Standard InChIKey | RVIGBTUDFAGRTQ-LLVKDONJSA-N | ||
| Standard InChI | InChI=1S/C14H19NO2/c1-3-14(16)15-11-8-7-10-5-4-6-13(17-2)12(10)9-11/h4-6,11H,3,7-9H2,1-2H3,(H,15,16)/t11-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Melatonin receptor agonist, 20-fold selective for the MT2 (vs MT1) subtype. At human recombinant MT1 and MT2 receptors, displays pKi values of 8.23 and 8.95 respectively. |

8-M-PDOT Dilution Calculator

8-M-PDOT Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2861 mL | 21.4307 mL | 42.8614 mL | 85.7229 mL | 107.1536 mL |
| 5 mM | 0.8572 mL | 4.2861 mL | 8.5723 mL | 17.1446 mL | 21.4307 mL |
| 10 mM | 0.4286 mL | 2.1431 mL | 4.2861 mL | 8.5723 mL | 10.7154 mL |
| 50 mM | 0.0857 mL | 0.4286 mL | 0.8572 mL | 1.7145 mL | 2.1431 mL |
| 100 mM | 0.0429 mL | 0.2143 mL | 0.4286 mL | 0.8572 mL | 1.0715 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6''-O-Acetylglycitin
Catalog No.:BCN3866
CAS No.:134859-96-4
- A 412997 dihydrochloride
Catalog No.:BCC6224
CAS No.:1347744-96-0
- Linderane
Catalog No.:BCN5023
CAS No.:13476-25-0
- Lamivudine
Catalog No.:BCC3801
CAS No.:134678-17-4
- GSK343
Catalog No.:BCC1607
CAS No.:1346704-33-3
- GSK621
Catalog No.:BCC6517
CAS No.:1346607-05-3
- GSK126
Catalog No.:BCC1604
CAS No.:1346574-57-9
- GSK503
Catalog No.:BCC6386
CAS No.:1346572-63-1
- ML 240
Catalog No.:BCC5604
CAS No.:1346527-98-7
- Zinc Pyrithione
Catalog No.:BCC5008
CAS No.:13463-41-7
- Eriocitrin
Catalog No.:BCN1208
CAS No.:13463-28-0
- Stavudine sodium
Catalog No.:BCC4263
CAS No.:134624-73-0
- 4-P-PDOT
Catalog No.:BCC6900
CAS No.:134865-74-0
- SAR245409
Catalog No.:BCC2534
CAS No.:934493-76-2
- Taccalonolide AJ
Catalog No.:BCN2971
CAS No.:1349904-82-0
- 2,2'-Dithiobisbenzanilide
Catalog No.:BCC8497
CAS No.:135-57-9
- Pseudotropine
Catalog No.:BCN1932
CAS No.:135-97-7
- Z-Hyp-OH
Catalog No.:BCC3257
CAS No.:13504-85-3
- Clopidogrel bisulfate
Catalog No.:BCC8917
CAS No.:135046-48-9
- MK-5172 hydrate
Catalog No.:BCC1763
CAS No.:1350462-55-3
- MK-5172
Catalog No.:BCC1762
CAS No.:1350514-68-9
- Repaglinide
Catalog No.:BCC2504
CAS No.:135062-02-1
- kb-NB77-78
Catalog No.:BCC5462
CAS No.:1350622-33-1
- Camelliaside A
Catalog No.:BCN3871
CAS No.:135095-52-2
Melatonin Suppresses Neuropathic Pain via MT2-Dependent and -Independent Pathways in Dorsal Root Ganglia Neurons of Mice.[Pubmed:28656058]
Theranostics. 2017 May 12;7(7):2015-2032.
Melatonin (Mel) and its receptors (MT1 and MT2) have a well-documented efficacy in treating different pain conditions. However, the anti-nociceptive effects of Mel and Mel receptors in neuropathic pain (NP) are poorly understood. To elucidate this process, pain behaviors were measured in a dorsal root ganglia (DRG)-friendly sciatic nerve cuffing model. We detected up-regulation of MT2 expression in the DRGs of cuff-implanted mice and its activation by the agonist 8-M-PDOT (8MP). Also, Mel attenuated the mechanical and thermal allodynia induced by cuff implantation. Immunohistochemical analysis demonstrated the expression of MT2 in the DRG neurons, while MT1 was expressed in the satellite cells. In cultured primary neurons, microarray analysis and gene knockdown experiments demonstrated that MT2 activation by 8MP or Mel suppressed calcium signaling pathways via MAPK1, which were blocked by RAR-related orphan receptor alpha (RORalpha) activation with a high dose of Mel. Furthermore, expression of nitric oxide synthase 1 (NOS1) was down-regulated upon Mel treatment regardless of MT2 or RORalpha. Application of Mel or 8MP in cuff-implanted models inhibited the activation of peptidergic neurons and neuro-inflammation in the DRGs by down-regulating c-fos, calcitonin gene-related peptide [CGRP], and tumor necrosis factor-1alpha [TNF-1alpha] and interleukin-1beta [IL-1beta]. Addition of the MT2 antagonist luzindole blocked the effects of 8MP but not those of Mel. In conclusion, only MT2 was expressed in the DRG neurons and up-regulated upon cuff implantation. The analgesic effects of Mel in cuff-implanted mice were closely associated with both MT2-dependent (MAPK-calcium channels) and MT2-independent (NOS1) pathways in the DRG.
REM sleep deprivation promotes a dopaminergic influence in the striatal MT2 anxiolytic-like effects.[Pubmed:27226821]
Sleep Sci. 2016 Jan-Mar;9(1):47-54.
The aim of this study was to investigate the possible anxiolytic-like effects of striatal MT2 activation, and its counteraction induced by the selective blockade of this receptor. Furthermore, we analyzed this condition under the paradigm of rapid eye movement (REM) sleep deprivation (REMSD) and the animal model of Parkinson's disease (PD) induced by rotenone. Male Wistar rats were infused with intranigral rotenone (12 mug/muL), and 7 days later were subjected to 24 h of REMSD. Afterwards the rats underwent striatal micro-infusions of selective melatonin MT2 receptor agonist, 8-M-PDOT (10 mug/muL) or selective melatonin MT2 receptor antagonist, 4-P-PDOT (5 mug/muL) or vehicle. Subsequently, the animals were tested in the open-field (OP) and elevated plus maze (EPM) tests. Results indicated that the activation of MT2 receptors produced anxiolytic-like effects. In opposite, the MT2 blockade did not show an anxiogenic-like effect. Besides, REMSD induced anxiolytic-like effects similar to 8-M-PDOT. MT2 activation generated a prevalent locomotor increase compared to MT2 blockade in the context of REMSD. Together, these results suggest a striatal MT2 modulation associated to the REMSD-induced dopaminergic supersensitivity causing a possible dopaminergic influence in the MT2 anxiolytic-like effects in the intranigral rotenone model of PD.
Putative role of monoamines in the antidepressant-like mechanism induced by striatal MT2 blockade.[Pubmed:25218873]
Behav Brain Res. 2014 Dec 15;275:136-45.
It has been observed that the secretion pattern of melatonin is modified in Parkinson's disease (PD). Hence, it is hypothesized that dysregulations of melatonin MT2 receptors may be involved in the installation of depression in PD patients. Together with recent evidence based on the use of the intranigral rotenone model of PD, have led to the hypothesis that modulating the striatal MT2 receptor could provide a more comprehensive understanding of the antidepressant properties triggered. To further investigate this issue, male Wistar rats were infused with intranigral rotenone (12mug/muL) and seven days later subjected to a rapid eye movement sleep deprivation (REMSD) for 24h. After, we injected within the striatum the MT2 selective agonist, 8-M-PDOT (10mug/muL), the MT2 selective antagonist, 4-P-PDOT (5mug/muL) or vehicle. Subsequently, they were tested in the forced swimming test and were allowed to perform the sleep rebound (REB). Then, the rats were re-tested, and the striatum, hippocampus and substantia nigra pars compacta (SNpc) were collected for neurochemical purposes. Results indicated substantial antidepressant effects promoted by the blockade of striatal MT2 receptors that were potentiated by REMSD. MT2 activation increased DA levels in the striatum and hippocampus, while MT2 blockade increase DA in the SNpc. 4-P-PDOT treatment of the rotenone REMSD group generated a decrement in 5-HT levels within the striatum, hippocampus and SNpc. However, increased 5-HT turnover was observed among these structures. Therefore, we demonstrated the neurochemical antidepressant effect induced by striatal MT2 blockage associated with REMSD in the rotenone model of PD.
MT2-like melatonin receptor modulates amplitude receptor potential in visual cells of crayfish during a 24-hour cycle.[Pubmed:19666131]
Comp Biochem Physiol A Mol Integr Physiol. 2009 Dec;154(4):486-92.
Retinular photoreceptors are structures involved in the expression and synchronization of the circadian rhythm of sensitivity to light in crayfish. To determine whether melatonin possesses a differential effect upon the receptor potential (RP) amplitude of retinular photoreceptors circadian time (CT)-dependent, we conducted experiments by means of applying melatonin every 2h during a 24-hour cycle. Melatonin with 100 nM increased RP amplitude during subjective day to a greater degree than during subjective night. To determine whether MT(2) melatonin receptors regulate the melatonin-produced effect, we carried out two experiments, circadian times (CTs) 6 and 18, which included the following: (1) application of the MT(2) receptor selective agonist 8-M-PDOT and antagonist DH97, and (2) the specific binding of [(125)I]-melatonin in eyestalk membranes. The amount of 10 nM of 8-M-PDOT increased RP amplitude in a similar manner to melatonin, and 1 nM DH97 abolished the increase produced by melatonin and 8-M-PDOT. Binding of [(125)I]-melatonin was saturable and specific. Scatchard analysis revealed an affinity constant (K(d)) of 1.1 nM and a total number of binding sites (B(max)) of 6 fmol/mg protein at CT 6, and a K(d) of 1.46 nM and B(max) of 7 fmol/mg protein at CT 18. Our results indicate that melatonin increased RP amplitude of crayfish retinular photoreceptors through MT(2)-like melatonin receptors. These data support the idea that melatonin is a signal of darkness for the circadian system in crayfish retinular cells.
Pharmacological characterization of human recombinant melatonin mt(1) and MT(2) receptors.[Pubmed:10696085]
Br J Pharmacol. 2000 Mar;129(5):877-86.
We have pharmacologically characterized recombinant human mt(1) and MT(2) receptors, stably expressed in Chinese hamster ovary cells (CHO-mt(1) and CHO-MT(2)), by measurement of [(3)H]-melatonin binding and forskolin-stimulated cyclic AMP (cAMP) production. [3H]-melatonin bound to mt(1) and MT(2) receptors with pK(D) values of 9.89 and 9.56 and B(max) values of 1.20 and 0.82 pmol mg(-1) protein, respectively. Whilst most melatonin receptor agonists had similar affinities for mt(1) and MT(2) receptors, a number of putative antagonists had substantially higher affinities for MT(2) receptors, including luzindole (11 fold), GR128107 (23 fold) and 4-P-PDOT (61 fold). In both CHO-mt(1) and CHO-MT(2) cells, melatonin inhibited forskolin-stimulated accumulation of cyclic AMP in a concentration-dependent manner (pIC(50) 9.53 and 9.74, respectively) causing 83 and 64% inhibition of cyclic AMP production at 100 nM, respectively. The potencies of a range of melatonin receptor agonists were determined. At MT(2) receptors, melatonin, 2-iodomelatonin and 6-chloromelatonin were essentially equipotent, whilst at the mt(1) receptor these agonists gave the rank order of potency of 2-iodomelatonin>melatonin>6-chloromelatonin. In both CHO-mt(1) and CHO-MT(2) cells, melatonin-induced inhibition of forskolin-stimulated cyclic AMP production was antagonized in a concentration-dependent manner by the melatonin receptor antagonist luzindole, with pA(2) values of 5.75 and 7.64, respectively. Melatonin-mediated responses were abolished by pre-treatment of cells with pertussis toxin, consistent with activation of G(i)/G(o) G-proteins. This is the first report of the use of [(3)H]-melatonin for the characterization of recombinant mt(1) and MT(2) receptors. Our results demonstrate that these receptor subtypes have distinct pharmacological profiles.
Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor.[Pubmed:9089668]
Naunyn Schmiedebergs Arch Pharmacol. 1997 Mar;355(3):365-75.
We have identified subtype selective agonists, partial agonists and antagonists, which distinguish the human recombinant Mel1a and Mel1b melatonin receptors expressed in COS-7 cells. Melatonin receptor agonists showed higher affinity for competition of 2-[125I]-iodomelatonin binding for the Mel1b than the Mel1a melatonin receptor. The dissociation constants (Ki) of 16 agonists determined on the recombinant human Mel1a and Mel1b melatonin receptor subtypes showed a significant correlation (r2 = 0.85, slope = 0.97, P < 0.0001, n = 16). However, six agonists showed 10 to 60 fold higher affinity for the Mel1b melatonin receptor as indicated by the affinity selectivity ratios (Mel1a/Mel1b) [8-methoxy-2-acetamidotetraline (11); S20098 (14); 8-methoxy-2-propionamidotetraline (20); 6, 7 di-chloro-2-methylmelatonin (21); 6-chloromelatonin (57); 6-methoxymelatonin (59)]. Dissociation constants for competition of 11 partial agonists and antagonist for 2-[125I]-iodomelatonin binding were between 15.5 (luzindole, pKi: 7.7) to 362 (4-phenyl-2-chloroacetamidotetraline, pKi: 9.1) fold higher for the Mel1b than for the Mel1a melatonin receptor. The lack of correlation between the pKi values (r2 = 0.23, P > 0.1, n = 11) strongly suggest that the two human melatonin receptor subtypes can be distinguished pharmacologically. The partial agonist: 5-methoxyluzindole (pKi: 9.6) and the competitive melatonin receptor antagonists: GR128107 (pKi: 9.6), 4-phenyl-2-chloroacetamidotetraline (pKi: 9.1), 4-phenyl-2-acetamidotetraline (pKi: 8.9) and 4-phenyl-2-propionamidotetraline (pKi: 8.8) are selective Mel1b melatonin receptor analogues as their affinity selectivity ratios (Mel1a/Mel1b) are bigger than 100. We conclude that the 40% overall amino acid difference in the sequence of the human recombinant Mel1a and Mel1b melatonin receptors is reflected in distinct pharmacological profiles for the subtypes. We compared the pharmacological profile of the presynaptic ML1 melatonin heteroreceptor of rabbit retina mediating inhibition of the calcium-dependent release of dopamine to that of the recombinant Mel1a and Mel1b melatonin receptors. Melatonin inhibited [3H]dopamine release by 50% (1C50) at 20 pM with a maximal inhibitory effect (80%) at 1 nM. The partial agonists, i.e., N-acetyltryptamine (1C50 5.6, maximal inhibition 55%) and 5-methoxyluzindole (1C50: 1.3, maximal inhibition 40%) showed various degrees of efficacy while none of the competitive melatonin receptor antagonists did inhibit [3H]dopamine release on their own. The potency (1C50) of full melatonin receptor agonists significantly correlated with their affinity to compete for 2-[125I]-iodomelatonin binding to either the Mel1a (r2 = 0.76, slope = 0.77, P < 0.0001, n = 17) or Mel1b (r2 = 0.63, slope = 0.75, P < 0.001, n = 17) human melatonin receptors. By contrast, the apparent dissociation constants (KB) for partial agonists and antagonists to antagonize the inhibition of [3H]dopamine release mediated by activation of the ML1 heteroreceptor by melatonin, significantly correlated with the affinity constants (Ki) for 2-[125I]-iodomelatonin binding determined of the Mel1b (r2 = 0.77, slope = 0.55, P < 0.001; n = 11) but not the Mel1a (r2 = 0.27, P < 0.1, n = 11) subtype. Together these results demonstrate that the pharmacological profile of the human recombinant Mel1b melatonin receptor is similar to that of the functional presynaptic melatonin heteroreceptor of rabbit retina, which we referred as an ML1B subtype. We conclude that the selective Mel1b melatonin partial agonists and antagonists described here can be used to identify melatonin receptor subtypes in native tissues and to search for subtype selective analogues with therapeutic potential.
Melatonin receptors: are there multiple subtypes?[Pubmed:7762083]
Trends Pharmacol Sci. 1995 Feb;16(2):50-6.
There is now evidence for more than one site of action for the hormone melatonin (N-acetyl-5-methoxy-tryptamine). Recent pharmacological and molecular advances are providing the tools to address the characterization of melatonin receptor subtypes. The development of novel melatonin receptor agonists and antagonists, high-affinity radioligands, quantitative bioassays, and the recent cloning of melatonin receptors are furthering our understanding of native and recombinant melatonin receptors. In this article, Margarita Dubocovich discusses the properties of melatonin receptors, and the basis for their classification into at least two subtypes, the ML1 and ML2.


