LactoseCAS# 14641-93-1 |
- Maltose
Catalog No.:BCC8338
CAS No.:69-79-4
Quality Control & MSDS
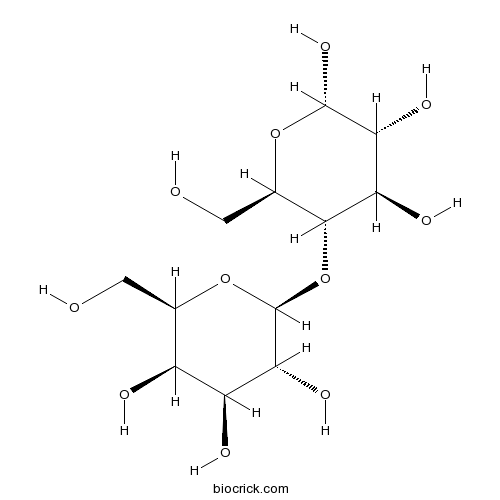
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14641-93-1 | SDF | Download SDF |
| PubChem ID | 84571 | Appearance | White crystalline powder |
| Formula | C12H22O11 | M.Wt | 342.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 63-42-3 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R,4S,5R,6S)-2-(hydroxymethyl)-6-[(2R,3S,4R,5R,6S)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxane-3,4,5-triol | ||
| SMILES | C(C1C(C(C(C(O1)OC2C(OC(C(C2O)O)O)CO)O)O)O)O | ||
| Standard InChIKey | GUBGYTABKSRVRQ-XLOQQCSPSA-N | ||
| Standard InChI | InChI=1S/C12H22O11/c13-1-3-5(15)6(16)9(19)12(22-3)23-10-4(2-14)21-11(20)8(18)7(10)17/h3-20H,1-2H2/t3-,4-,5+,6+,7-,8-,9-,10-,11+,12+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Lactose Dilution Calculator

Lactose Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9214 mL | 14.6071 mL | 29.2141 mL | 58.4283 mL | 73.0353 mL |
| 5 mM | 0.5843 mL | 2.9214 mL | 5.8428 mL | 11.6857 mL | 14.6071 mL |
| 10 mM | 0.2921 mL | 1.4607 mL | 2.9214 mL | 5.8428 mL | 7.3035 mL |
| 50 mM | 0.0584 mL | 0.2921 mL | 0.5843 mL | 1.1686 mL | 1.4607 mL |
| 100 mM | 0.0292 mL | 0.1461 mL | 0.2921 mL | 0.5843 mL | 0.7304 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Desmethylrocaglamide
Catalog No.:BCN7735
CAS No.:146408-78-8
- SR 48692
Catalog No.:BCC7763
CAS No.:146362-70-1
- N-Methyllidocaine iodide
Catalog No.:BCC6905
CAS No.:1462-71-1
- Chlorajapolide F
Catalog No.:BCN6425
CAS No.:1461760-59-7
- R-(-)-Deprenyl hydrochloride
Catalog No.:BCC5196
CAS No.:14611-52-0
- Z-Arg(Z)2-OH
Catalog No.:BCC3574
CAS No.:14611-34-8
- Pulchinenoside E1
Catalog No.:BCN8185
CAS No.:146100-02-9
- Dihydromarein
Catalog No.:BCN8406
CAS No.:
- MSDC-0160
Catalog No.:BCC5343
CAS No.:146062-49-9
- Tauroursodeoxycholic acid
Catalog No.:BCN6953
CAS No.:14605-22-2
- SC 51089
Catalog No.:BCC7773
CAS No.:146033-02-5
- SC 51322
Catalog No.:BCC5941
CAS No.:146032-79-3
- Flavopiridol
Catalog No.:BCC1577
CAS No.:146426-40-6
- Camaric acid
Catalog No.:BCN1650
CAS No.:146450-83-1
- Pralatrexate
Catalog No.:BCC2304
CAS No.:146464-95-1
- 1-Methylpsilocin
Catalog No.:BCC7536
CAS No.:1465-16-3
- Complanatoside A
Catalog No.:BCN6282
CAS No.:146501-37-3
- WR 1065
Catalog No.:BCC2417
CAS No.:14653-77-1
- Tyrphostin AG 1296
Catalog No.:BCC1195
CAS No.:146535-11-7
- Fmoc-Gly(allyl)-OH
Catalog No.:BCC3156
CAS No.:146549-21-5
- 2-Cyclopropyl-3-[(diphenylphosphinyl)methyl]-4-(4-fluorophenyl)quinoline
Catalog No.:BCC8572
CAS No.:146578-99-6
- 2,6-Dimethoxybenzoic acid
Catalog No.:BCN1651
CAS No.:1466-76-8
- Dantrolene, sodium salt
Catalog No.:BCC6673
CAS No.:14663-23-1
- H-Trp(Boc)-OH
Catalog No.:BCC3115
CAS No.:146645-63-8
Production of calcium- and magnesium-enriched caseins and caseinates by an ecofriendly technology.[Pubmed:29753479]
J Dairy Sci. 2018 Aug;101(8):7002-7012.
Finding new environmentally friendly ways of producing proteins has never been of such critical public interest, both to meet consumers' needs and to preserve the environment. Milk proteins are among the most attractive protein types due to their high nutritional value and attractive functional properties. In this work, the separation of caseins by conventional chemical acidification was compared with electrodialysis with bipolar membrane coupled to an ultrafiltration module (EDBM-UF), a green process that allows the precipitation of caseins by H(+) generated in situ by the bipolar membrane and, simultaneously, the production of a separated NaOH stream from OH(-) electrogenerated by the bipolar membrane. Caseinate production using this NaOH stream by-product and the quantity of NaOH needed to produce caseinates from both methods were also investigated. Hence, the purity and composition of caseins and caseinates were compared in terms of protein, ash, and Lactose contents as well as mineral composition. The results showed for the first time that caseinates can be produced by solubilizing caseins with NaOH stream from the EDBM process. Furthermore, the caseins and caseinates produced by EDBM-UF were equivalent in terms of Lactose and protein contents to their respective caseins and caseinates that were chemically produced but presented slightly lower sodium content and 3 to 4 times higher magnesium and calcium contents. The fact that calcium and magnesium are likely bound to milk caseins would ensure their favorable absorbability. These caseins or caseinates from the new EDBM-UF process could be suitable as an improved protein-based calcium or magnesium supplement, both for their enhanced nutritional quality and because they are produced by a "green" process.
Responses of milk production of dairy cows to jugular infusions of a mixture of essential amino acids with or without exclusion leucine or arginine.[Pubmed:29767155]
Anim Nutr. 2017 Sep;3(3):271-275.
The purpose of this study was to determine effects of jugular infusion of either balanced or imbalanced amino acid mixture on milk production and composition in dairy cows. Eight mid-lactation Holstein cows were randomly assigned to 5-d continuous jugular infusions of saline (CTL), essential amino acid (EAA) mixture prepared on the profile of casein (CSN, 160 g/d), EAA mixture excluding leucine (Leu) (-Leu, 163 g/d) or EAA mixture excluding arginine (Arg) (-Arg, 158 g/d) in a duplicated 4 x 4 Latin square design with 4 infusion periods separated by a 7-d interval period. The basal diet was formulated with corn grain, soybean meal, cottonseed meal, corn silage, alfalfa hay and Chinese wildrye grass hay according to NRC (2001) and supplied 1.6 Mcal net energy for lactation (NEL) and 94.4 g metabolizable protein (MP) per kg dry matter (DM) to meet requirements for lactation. The results showed that the dry matter intake (DMI) and normal physiological status were not affected by amino acid mixture infusions. Compared with CTL treatment, the CSN treatment increased milk yield (14.9%, P < 0.001), milk Lactose yield (14.5%, P = 0.001), milk fat yield (16.6%, P = 0.01), milk protein yield (18.2%, P < 0.001) and the contents of alphaS1-casein (alphaS1-CN, 11.8%, P = 0.007), beta-casein (beta-CN, 4.2%, P = 0.035) and kappa-casein (kappa-CN, 8.5%, P = 0.003). However, the -Leu and -Arg treatments had lower milk yield (6.3%, P = 0.058 and 5.7%, P = 0.073, respectively), milk protein yield (8.8%, P = 0.010 and 8.2%, P = 0.011, respectively) and the contents of alphaS1-CN (7.3%, P = 0.057 and 8.4%, P = 0.026, respectively), beta-CN (4.2%, P = 0.033 and 3.8%, P = 0.048, respectively) and kappa-CN (5.8%, P = 0.023 and 7.6%, P = 0.003, respectively) than those of the CSN treatment. Milk Lactose yield (5.9%, P = 0.076) tended to decrease when Leu was removed from amino acid mixture infusate. In conclusion, the supply of casein profile can increase milk production in dairy cows, but a deficiency of Leu or Arg had negative effects on milk yield and milk protein yield.
Wavelength Modulated Back-Scatter Interferometry for Universal, On-Column Refractive Index Detection in Picoliter Volumes.[Pubmed:29762009]
Anal Chem. 2018 Jun 5;90(11):6789-6795.
Wavelength-modulated back scatter interferometry (M-BSI) is shown to improve the detection metrics for refractive index (RI) sensing in microseparations. In M-BSI, the output of a tunable diode laser is focused into the detection zone of a separation channel as the excitation wavelength is rapidly modulated. This spatially modulates the observed interference pattern, which is measured in the backscattered direction. Phase-sensitive detection using a split photodiode detector aligned on one fringe of the interference pattern is used to monitor RI changes as analytes are separated. Using sucrose standards, we report a detection limit of 700 mug/L in a 75 mum i.d. capillary at the 3sigma level, corresponding to a detection volume of 90 pL. To validate the approach for electrophoretic separations, Na(+) and Li(+) were separated and detected with M-BSI and indirect-UV absorbance on the same capillary. A 4 mg/L NaCl and LiCl mixture leads to comparable separation efficiencies in the two detection schemes, with better signal-to-noise in the M-BSI detection, but less baseline stability. The latter arises in part from Joule heating, which influences RI measurements through the thermo-optic properties of the run buffer. To reduce this effect, a 25 mum i.d. capillary combined with active temperature control was used to detect the separation of sucrose, glucose, and Lactose with M-BSI. The lack of suitable UV chromophores makes these analytes challenging to detect directly in ultrasmall volumes. Using a 55 mM NaOH run buffer, M-BSI is shown to detect the separation of a mixture of 174 mg/L sucrose, 97 mg/L glucose, and 172 mg/L Lactose in a 15 pL detection volume. The universal on-column detection in ultrasmall volumes adds new capabilities for microanalysis platforms, while potentially reducing the footprint and costs of these systems.
A comparative study of the influence of alpha-lactose monohydrate particle morphology on granule and tablet properties after roll compaction/dry granulation.[Pubmed:29757067]
Pharm Dev Technol. 2019 Mar;24(3):314-322.
The influence of particle morphology and size of alpha-Lactose monohydrate on dry granules and tablets was studied. Four different morphologies were investigated: Two grades of primary crystals, which differed in their particle size and structure (compact crystals vs. agglomerates). The materials were roll compacted at different specific compaction forces and changes in the particle size distribution and the specific surface area were measured. Afterwards, two fractions of granules were pressed to tablets and the tensile strength was compared to that from tablets compressed from the raw materials. The specific surface area was increased induced by roll compaction/dry granulation for all materials. At increased specific compaction forces, the materials showed sufficient size enlargement. The morphology of Lactose determined the strength of direct compressed tablets. In contrast, the strength of granule tablets was leveled by the previous compression step during roll compaction/dry granulation. Thus, the tensile strength of tablets compressed directly from the powder mixtures determined whether materials exhibited a loss in tabletability after roll compaction/dry granulation or not. The granule size had only a slight influence on the strength of produced tablets. In some cases, the fraction of smaller granules showed a higher tensile strength compared to the larger fraction.
Use of Starch and Modified Starches in Infant Feeding: A Historical Perspective.[Pubmed:29762373]
J Pediatr Gastroenterol Nutr. 2018 Jun;66 Suppl 3:S30-S34.
There is a long history of the use of starch in infant feeding. Proprietary infant foods (1867-1920) contained added starch from either cereal grains or malted carbohydrates. When evaporated milk became available in the 1920s, the use of proprietary foods fell out of favor. Evaporated milk formulas were a mixture of milk, water, and modified starch or milk sugar (Lactose). By the late 1920s, however, corn syrup became the most common modified starch added to evaporated milk formulas as it was widely available, inexpensive, and readily accepted. The ongoing development of the modern calorie-based infant formula, made from non-fat cow's milk, Lactose, oleo and vegetable oils, largely replaced the evaporated milk formulas in the 1960s. On the other hand, after 1940, added starch and modified starch became increasingly important in the production of pureed fruits and vegetables. Not surprisingly, this included their use in the modern "industrialized" food for use in infants, including their use in a proliferation of grain based fortified infant cereals. This coincided with the increasing production largely due to the earlier introduction of complementary foods, commonly before 3 months of age by 1958. After 1969, the increasing public awareness and media scrutiny of infant foods lead to a growing criticism of the use of modified starches. Even though the National Research Council and the American Academy of Pediatrics concluded that modified starches were safe for use, continued public pressure led to their removal from most infant foods in the 1990s. This paralleled the natural food and organic food movements in the United States. Though modified starches are still used in infant dinners of mixed foods today, their use has been minimized and this issue is not currently of significant concern to the public.


