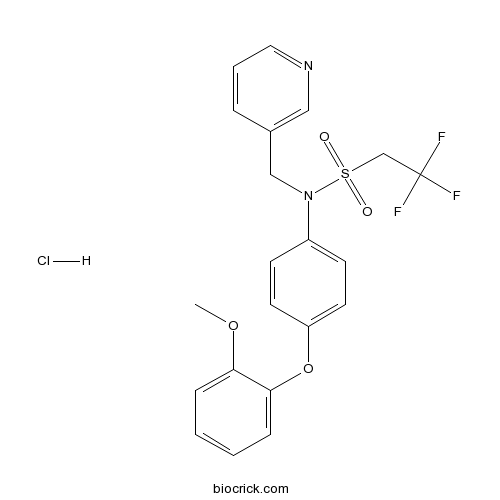
LY 487379 hydrochloridePositive allosteric modulator selective for mGlu2 CAS# 353229-59-1 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- BMS 299897
Catalog No.:BCC2340
CAS No.:290315-45-6
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- Begacestat
Catalog No.:BCC2346
CAS No.:769169-27-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 353229-59-1 | SDF | Download SDF |
| PubChem ID | 56972206 | Appearance | Powder |
| Formula | C21H20ClF3N2O4S | M.Wt | 488.91 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 50 mM in ethanol | ||
| Chemical Name | 2,2,2-trifluoro-N-[4-(2-methoxyphenoxy)phenyl]-N-(pyridin-3-ylmethyl)ethanesulfonamide;hydrochloride | ||
| SMILES | COC1=CC=CC=C1OC2=CC=C(C=C2)N(CC3=CN=CC=C3)S(=O)(=O)CC(F)(F)F.Cl | ||
| Standard InChIKey | LPWFRDWTOKLHJC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H19F3N2O4S.ClH/c1-29-19-6-2-3-7-20(19)30-18-10-8-17(9-11-18)26(14-16-5-4-12-25-13-16)31(27,28)15-21(22,23)24;/h2-13H,14-15H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Positive allosteric modulator selective for mGlu2 receptors. Potentiates glutamate-stimulated [35S]GTPγS binding (EC50 values are 1.7 and > 10 μM for mGlu2 and mGlu3 receptors respectively). Devoid of any activity at mGlu5 and mGlu7 receptors. Promotes cognitive flexibility in a rat model. |

LY 487379 hydrochloride Dilution Calculator

LY 487379 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0454 mL | 10.2268 mL | 20.4537 mL | 40.9073 mL | 51.1342 mL |
| 5 mM | 0.4091 mL | 2.0454 mL | 4.0907 mL | 8.1815 mL | 10.2268 mL |
| 10 mM | 0.2045 mL | 1.0227 mL | 2.0454 mL | 4.0907 mL | 5.1134 mL |
| 50 mM | 0.0409 mL | 0.2045 mL | 0.4091 mL | 0.8181 mL | 1.0227 mL |
| 100 mM | 0.0205 mL | 0.1023 mL | 0.2045 mL | 0.4091 mL | 0.5113 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- D-Alaninol
Catalog No.:BCC2728
CAS No.:35320-23-1
- (E)-3-Hydroxy-5-methoxystilbene
Catalog No.:BCN5292
CAS No.:35302-70-6
- Ziyuglycoside II
Catalog No.:BCN5291
CAS No.:35286-59-0
- Ziyuglycoside I
Catalog No.:BCN5290
CAS No.:35286-58-9
- 1-Chloroindan
Catalog No.:BCN2244
CAS No.:35275-62-8
- Caesalmin B
Catalog No.:BCN7252
CAS No.:352658-23-2
- Delafloxacin meglumine
Catalog No.:BCC1523
CAS No.:352458-37-8
- 9-Methoxy-alpha-lapachone
Catalog No.:BCN5289
CAS No.:35241-80-6
- Benzoin methyl ether
Catalog No.:BCC8857
CAS No.:3524-62-7
- 4-(Bromomethyl)-7-methoxy coumarin
Catalog No.:BCC9202
CAS No.:35231-44-8
- Alloisoimperatorin
Catalog No.:BCN6789
CAS No.:35214-83-6
- Neobyakangelicol
Catalog No.:BCN5288
CAS No.:35214-82-5
- NS 6180
Catalog No.:BCC6307
CAS No.:353262-04-1
- S26948
Catalog No.:BCC7751
CAS No.:353280-43-0
- Monocrotaline N-oxide
Catalog No.:BCN2097
CAS No.:35337-98-5
- TQS
Catalog No.:BCC7896
CAS No.:353483-92-8
- 9-Hydroxycalabaxanthone
Catalog No.:BCN5293
CAS No.:35349-68-9
- HLM006474
Catalog No.:BCC5403
CAS No.:353519-63-8
- Honokiol
Catalog No.:BCN1001
CAS No.:35354-74-6
- J 113863
Catalog No.:BCC7422
CAS No.:353791-85-2
- Norathyriol
Catalog No.:BCN5294
CAS No.:3542-72-1
- 1-Methyl-5-nitro-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8470
CAS No.:3543-72-4
- 1-Methyl-5-amino-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8469
CAS No.:3543-73-5
- 5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8724
CAS No.:3543-74-6
[Phase I study of gemcitabine hydrochloride (LY 188011) combination therapy with cisplatin in the patients with non-small cell lung cancer].[Pubmed:10396316]
Gan To Kagaku Ryoho. 1999 Jun;26(7):898-907.
The combination Phase I study of gemcitabine hydrochloride with cisplatin was conducted in the patients with non-small cell lung cancer (NSCLC) at 5 investigation sites. Gemcitabine was administrated on day 1, 8 and 15 and cisplatin on day 1 of each 28-day cycle. The dosage of cisplatin was fixed to 80 mg/m2 and the dosage of Gemcitabine was gradually escalated in 3 dosing level from 600, 800 to 1,000 mg/m2. The maximum tolerated dose (MTD) and the recommended dose was determined with Continual Reassessment Method. For each dose level, 6 cases, 3 cases and 6 cases were registered respectively and all 15 cases were evaluable. In the dose level 3 with 1,000 mg/m2 of gemcitabine and 80 mg/m2 of cisplatin, grade 4 neutropenia was observed as DLT in 3 out of 6 cases, thus dose level 3 was considered as MTD and the recommended dose. Major adverse events were leukopenia, neutropenia, nausea/vomiting and anorexia. The incidence of such adverse events seemed to be dose-dependent and especially the grade of neutropenia seemed to be more serious as the dose increased. Also, the grade of liver function tests abnormal seemed to be more serious as the dose increased but the incidence as well as the grade did not have tendency of dose-dependent in another events including renal function tests abnormal. On the other hand, as to the efficacy PR was observed in 4 out of 15 cases. Based upon the results, it is necessary to discuss further the efficacy in the recommended dose in the combination therapy of gemcitabine and cisplatin.
[An early phase II study of gemcitabine hydrochloride (LY 188011). Gemcitabine Cooperative Study Group for Early Phase II].[Pubmed:8937492]
Gan To Kagaku Ryoho. 1996 Nov;23(13):1813-24.
An early phase II cooperative study of Gemcitabine Hydrochloride (abbreviated to "gemcitabine" herewith) was conducted in patients with a variety of solid tumors (i.e., lung cancer, gastric cancer, pancreatic cancer, colon/rectum cancer, cervical cancer, ovarian cancer and breast cancer) at 56 institutions. The aim of the first step (Step I) was to investigate the feasibility of gemcitabine in a variety of different solid tumors, including lung cancer regarding efficacy and safety. The aim of the second step (Step II) was as a result of step I (Responses were observed) to continue to investigate the efficacy and safety of gemcitabine in chemonaive patients with non-small cell lung cancer. As a Step I study, gemcitabine was administered once weekly at a dose of 800 mg/m2 for a consecutive 3-week period followed by a week of rest, in multiple courses. Among the 29 eligible patients with lung cancer, partial response (PR) was achieved in 3 patients (25.0%, 95% confidence interval: 5.5-57.2%) out of 12 chemonaive patients. Adverse reactions (grade 3 or higher) seen in 29 patients with lung cancer were neutropenia (27.6%), leukopenia (13.8%), decreased hemoglobin (13.8%), thrombocytopenia (10.3%), malaise (6.9%), anorexia (3.4%), nausea/vomiting (3.4%), diarrhea (3.4%), dyspnea (3.4%) and interstitial pneumonia (3.4%). In other types of solid tumors, PR was achieved in 2 (8.7%) out of 23 eligible patients with cervical cancer and in 1 (5.3%) of 19 eligible patients with ovarian cancer, while the use of analgesics became unnecessary in 1 patient with pancreatic cancer. Incidence as well as severity of main adverse reactions in these patients were comparable to those seen in patients with lung cancer. A Step II study, in which gemcitabine was administered once weekly at a dose of 1,000 mg/m2 to chemonaive patients with non-small cell lung cancer, was conducted, referring to the results of Step I and clinical studies conducted overseas. The results of the Step II study demonstrated PR in 5 (14.3%, 95% confidence interval: 4.8 - 30.3%) out of 35 eligible patients with non-small cell lung cancer and that the main adverse reactions were comparable to those seen in the Step I study, posing no tolerability problems in particular.
Changes in motor activities induced by microinjections of the selective dopamine agonists LY 171555, quinpirole hydrochloride, and SK&F 38393 into the habenula nucleus.[Pubmed:3495009]
Pharmacol Biochem Behav. 1987 Mar;26(3):643-6.
The effects on behaviour of microinjections into the habenula complex of selective agonists for dopamine D-1 (SK&F 38393) and D-2 (LY 171555) receptors were documented in a holeboard, open-field test. The D-2 agonist reduced grooming responses, locomotor activity and rearing behaviour. In contrast, the D-1 agonist increased rearing and locomotor activity but was without effect on grooming responses. Neither drug produced significant effects on inspective hole exploration. The data extend findings of behavioural consequences of central D-1 receptor activation and provide direct evidence in support of the functional and behavioural importance of intrahabenular dopamine receptor sites. The findings are consistent with suggestions for feedback regulation of habenular efferents to midbrain dopaminergic neurons. Effects of both receptor agonists on some responses but not others indicates potential complex interactions between D-1 and D-2 receptors within the habenula.
Effects of a positive allosteric modulator of group II metabotropic glutamate receptors, LY487379, on cognitive flexibility and impulsive-like responding in rats.[Pubmed:20739457]
J Pharmacol Exp Ther. 2010 Dec;335(3):665-73.
Orthosteric group II metabotropic glutamate receptor (mGluR) agonists are regarded as novel, effective medications for all major symptom domains of schizophrenia, including cognitive disturbances. mGluR2s also can be affected in a more subtle way by positive allosteric modulators (PAMs) characterized by a unique degree of subtype selectivity and neuronal frequency-dependent activity. Because currently available treatments for schizophrenia do not improve cognitive dysfunction, the main aim of the present study was to examine the effects of a mGluR2 PAM, N-(4-(2-methoxyphenoxy)-phenyl-N-(2,2,2-trifluoroethylsulfonyl)-pyrid-3-ylmethyla mine (LY487379), on rat cognitive flexibility and impulsive-like responding, assessed in an attentional set-shifting task (ASST) and a differential reinforcement of low-rate 72 s (DRL72) schedule of food reinforcement. In addition, in vivo microdialysis was used to assess the drug's impact on cortical levels of dopamine, norepinephrine, serotonin, and glutamate. Rats treated with LY487379 (30 mg/kg) required significantly fewer trials to criteria during the extradimensional shift phase of the ASST. Under a DRL72 schedule, LY487379 (30 mg/kg) decreased the response rate and increased the number of reinforcers obtained. These effects were accompanied by the shift of the frequency distribution of responses toward longer inter-response time durations. LY487379 significantly enhanced extracellular norepinephrine and serotonin levels in the medial prefrontal cortex. In summary, the present study demonstrates that a mGluR2 PAM, LY487379, promotes cognitive flexibility and facilitates behavioral inhibition. These procognitive effects may contribute to the therapeutic efficacy of agents stimulating mGluR2 in schizophrenia.
Metabotropic glutamate receptor 2 modulates excitatory synaptic transmission in the rat globus pallidus.[Pubmed:15993439]
Neuropharmacology. 2005;49 Suppl 1:57-69.
While group II metabotropic glutamate receptors (mGluRs) are known to be expressed in the rat globus pallidus (GP), their functions remain poorly understood. We used standard patch clamping technique in GP slices to determine the effect of group II mGluR activation on excitatory transmission in this region. Activation of group II mGluRs with the group-selective agonist DCG-IV or APDC reduced the amplitude of the evoked excitatory postsynaptic currents (EPSCs) and significantly increased the paired pulse ratio suggesting a presynaptic site of action. This was further supported by double-labeling electron microscopy data showing that group II mGluRs (mGluR2 and 3) immunoreactivity is localized in glutamatergic pre-terminal axons and terminals in the GP. Furthermore, we found that LY 487379, an mGluR2-specific allosteric modulator, significantly potentiated the inhibitory effect of DCG-IV on the excitatory transmission in the GP. Co-incubation with 30 microM LY 487379 increased the potency of DCG-IV about 10-fold in the GP. We were thus able to pharmacologically isolate the mGluR2-mediated function in the rat GP using an mGluR2-specific allosteric modulator. Therefore, our findings do not only shed light on the functions of group II mGluRs in the GP, they also illustrate the therapeutic potential of mGluR-targeting allosteric modulators in neurological disorders such as Parkinson's disease.
Discovery of allosteric potentiators for the metabotropic glutamate 2 receptor: synthesis and subtype selectivity of N-(4-(2-methoxyphenoxy)phenyl)-N-(2,2,2- trifluoroethylsulfonyl)pyrid-3-ylmethylamine.[Pubmed:12852748]
J Med Chem. 2003 Jul 17;46(15):3189-92.
This report describes recently discovered novel allosteric modulators of metabotropic glutamate2 (mGlu2) receptors. These pyridylmethylsulfonamides (e.g., 3) potentiate glutamate, shifting agonist potency by 2-fold. This effect was specific for mGlu2 (vs mGlu1,3-8 receptors). Also, 3 failed to potentiate a chimeric mGlu2/1 receptor, demonstrating the mGlu2 transmembrane region's critical involvement. In a fear-potentiated startle model, 3 showed anxiolytic activity that was prevented by mGlu2/3 antagonist pretreatment. Thus, these pyridylmethylsulfonamides represent the first mGlu2 receptor potentiators discovered.
Pharmacological characterization and identification of amino acids involved in the positive modulation of metabotropic glutamate receptor subtype 2.[Pubmed:14500736]
Mol Pharmacol. 2003 Oct;64(4):798-810.
In the present study, we describe the characterization of a positive allosteric modulator at metabotropic glutamate subtype 2 receptors (mGluR2). N-(4-(2-Methoxyphenoxy)-phenyl-N-(2,2,2-trifluoroethylsulfonyl)-pyrid-3-ylmethyla mine (LY487379) is a selective positive allosteric modulator at human mGluR2 and is without activity at human mGluR3. Furthermore, LY487379 has no intrinsic agonist or antagonist activity at hmGluR2, as determined by functional guanosine 5'(gamma-[35S]thio)triphosphate ([35S]GTPgammaS) binding, single-cell Ca2+ imaging, and electrophysiological studies. However, LY487379 markedly potentiated glutamate-stimulated [35S]GTPgammaS binding in a concentration-dependent manner at hmGluR2, shifting the glutamate dose-response curve leftward by 3-fold and increasing the maximum levels of [35S]GTPgammaS stimulation. This effect of LY487479 was also observed to a greater extent on the concentration-response curves to selective hmGluR2/3 agonists. In radioligand binding studies to rat cortical membranes, LY487379 increased the affinity of the radiolabeled agonist, [3H]DCG-IV, without affecting the binding affinity of the radiolabeled antagonist, [3H]LY341495. In rat hippocampal slices, coapplication of LY487379 potentiated synaptically evoked mGluR2 responses. Finally, to elucidate the site of action, we systematically exchanged segments and single amino acids between hmGluR2 and hmGluR3. Substitution of Ser688 and/or Gly689 in transmembrane IV along with Asn735 located in transmembrane segment V, with the homologous amino acids of hmGluR3, completely eliminated LY487379 allosteric modulation of hmGluR2. We propose that this allosteric binding site defines a pocket that is different from the orthosteric site located in the amino terminal domain.


