KinsenosideCAS# 151870-74-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 151870-74-5 | SDF | Download SDF |
| PubChem ID | 10422896 | Appearance | Powder |
| Formula | C10H16O8 | M.Wt | 264.2 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
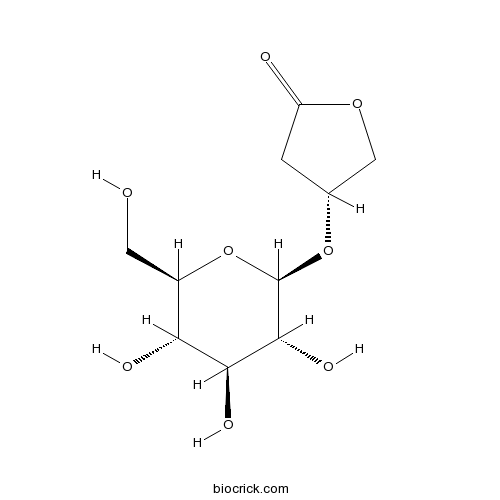
| Chemical Name | (4R)-4-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxolan-2-one | ||
| SMILES | C1C(COC1=O)OC2C(C(C(C(O2)CO)O)O)O | ||
| Standard InChIKey | MQEPWBMWFIVRPS-ZGSHZZHUSA-N | ||
| Standard InChI | InChI=1S/C10H16O8/c11-2-5-7(13)8(14)9(15)10(18-5)17-4-1-6(12)16-3-4/h4-5,7-11,13-15H,1-3H2/t4-,5-,7-,8+,9-,10-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Kinsenoside could be useful for repairing beta cells in pancreatic islet injury as well as improving its function, it could promote the glucose tolerance of acute glucose increase in both diabetic and normal healthy rats. 2. Kinsenoside shows significant antihepatotoxic activity. 3. Kinsenoside inhibits osteoclastogenesis from macrophages by attenuating RANKL-induced NF-κB and NFATc1 activities, which in turn, prevents bone loss from OVX mice. 4. Kinsenoside can inhibit the production of inflammatory mediators and enhanc anti-inflammatory cytokine generation, therefore, kinsenoside can alleviate acute inflammatory hazards. |
| Targets | NO | MMP(e.g.TIMP) | p65 | NF-kB | IkB | IL Receptor | TNF-α | IKK |

Kinsenoside Dilution Calculator

Kinsenoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.785 mL | 18.9251 mL | 37.8501 mL | 75.7002 mL | 94.6253 mL |
| 5 mM | 0.757 mL | 3.785 mL | 7.57 mL | 15.14 mL | 18.9251 mL |
| 10 mM | 0.3785 mL | 1.8925 mL | 3.785 mL | 7.57 mL | 9.4625 mL |
| 50 mM | 0.0757 mL | 0.3785 mL | 0.757 mL | 1.514 mL | 1.8925 mL |
| 100 mM | 0.0379 mL | 0.1893 mL | 0.3785 mL | 0.757 mL | 0.9463 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- cAMPS-Rp, triethylammonium salt
Catalog No.:BCC8082
CAS No.:151837-09-1
- AC 187
Catalog No.:BCC6018
CAS No.:151804-77-2
- Montelukast Sodium
Catalog No.:BCC4680
CAS No.:151767-02-1
- 4,4'-Dihydroxy-2,6-dimethoxydihydrochalcone
Catalog No.:BCN3583
CAS No.:151752-08-8
- 2,2-Dimethyl-8-prenylchromene 6-carboxylic acid
Catalog No.:BCN1675
CAS No.:151731-50-9
- 5-Deoxystrigol
Catalog No.:BCN7693
CAS No.:151716-18-6
- SEP-0372814
Catalog No.:BCC6429
CAS No.:1516895-53-6
- H-D-Ser-OBzl.HCl
Catalog No.:BCC3097
CAS No.:151651-44-4
- 6-Prenylquercetin-3-methylether
Catalog No.:BCN7992
CAS No.:151649-34-2
- XEN445
Catalog No.:BCC5382
CAS No.:1515856-92-4
- JJKK 048
Catalog No.:BCC5610
CAS No.:1515855-97-6
- ent-3-Oxokauran-17-oic acid
Catalog No.:BCN1674
CAS No.:151561-88-5
- 2-Phenylmelatonin
Catalog No.:BCC6748
CAS No.:151889-03-1
- 6- Methoxydihydrosanguinarine
Catalog No.:BCN8346
CAS No.:151890-26-5
- Ac2-26
Catalog No.:BCC5825
CAS No.:151988-33-9
- Verapamil HCl
Catalog No.:BCC4747
CAS No.:152-11-4
- Quinestrol
Catalog No.:BCC9132
CAS No.:152-43-2
- Vicine
Catalog No.:BCC8366
CAS No.:152-93-2
- Sophoricoside
Catalog No.:BCN2294
CAS No.:152-95-4
- Epothilone A
Catalog No.:BCC1091
CAS No.:152044-53-6
- Epothilone B (EPO906, Patupilone)
Catalog No.:BCC1092
CAS No.:152044-54-7
- Dp44mT
Catalog No.:BCC6518
CAS No.:152095-12-0
- 3,4-Dimethoxybenzamide
Catalog No.:BCN6565
CAS No.:1521-41-1
- 1,2-Epoxy-1-hydroxymethylpyrrolizidine
Catalog No.:BCN1557
CAS No.:15211-03-7
Antihyperglycemic activity of kinsenoside, a high yielding constituent from Anoectochilus roxburghii in streptozotocin diabetic rats.[Pubmed:17869039]
J Ethnopharmacol. 2007 Nov 1;114(2):141-5.
Different doses of Kinsenoside, a high yielding constituent from Anoectochilus roxburghii, was orally administered to further investigate its biological activity and pharmacological mechanisms that involve in the hypoglycemic effect on streptozotocin (STZ) diabetic rats. Our study showed that this compound exhibited significantly antihyperglycemic activity at the dose of 15mg/kg body weight, which is speculated to be partially attributed to modulating the activity of enzymatic antioxidants, scavenging free radicals, and reducing the content of factor NO. Much more intact beta cells in the islets of Langerhans with denser insulin in Kinsenoside-treated groups than the negative control were observed, which greatly supported the morphological and functional elucidation. These results displayed that Kinsenoside could be useful for repairing beta cells in pancreatic islet injury as well as improving its function. The OGTT evidenced that this compound could promote the glucose tolerance of acute glucose increase in both diabetic and normal healthy rats.
Kinsenoside isolated from Anoectochilus formosanus suppresses LPS-stimulated inflammatory reactions in macrophages and endotoxin shock in mice.[Pubmed:20661184]
Shock. 2011 Feb;35(2):184-90.
In the present study, we reported that Kinsenoside, a major component of Anoectochilus formosanus, inhibited inflammatory reactions in mouse peritoneal lavage macrophages and protects mice from endotoxin shock. In LPS-stimulated mouse peritoneal lavage macrophages, Kinsenoside inhibited the inflammatory mediators, such as nitric oxide, TNF-[alpha], IL-1[beta], monocyte chemoattractant protein 1, and macrophage migration inhibitory factor production. Furthermore, Kinsenoside decreased the formation of a nuclear factor [kappa]B-DNA complex and nuclear p65 and p50 protein levels. Kinsenoside inhibited nuclear factor [kappa]B translocation through both I[kappa]B[alpha]-dependent and -independent pathway. In contrast, it stimulated anti-inflammatory cytokine IL-10 generation and enhanced the mRNA expression of IL-10 and suppressor of cytokine signaling 3 in the same cells induced by LPS. In an animal model, both pretreatment and posttreatment of Kinsenoside increased the survival rate of ICR mice challenged by LPS (80 mg/kg, i.p.). Pretreatment with Kinsenoside decreased serum levels of TNF-[alpha], IL-1[beta], IL-10, monocyte chemoattractant protein 1, and migration inhibitory factor at 1 h after sublethal dose of LPS (40 mg/kg, i.p.) in mice. In contrast, Kinsenoside enhanced serum IL-10 level at 24 h after LPS injection in mice. In conclusion, Kinsenoside inhibited the production of inflammatory mediators and enhanced anti-inflammatory cytokine generation. Therefore, Kinsenoside can alleviate acute inflammatory hazards.
Kinsenoside prevents ovariectomy-induced bone loss and suppresses osteoclastogenesis by regulating classical NF-kappaB pathways.[Pubmed:23143538]
Osteoporos Int. 2013 May;24(5):1663-76.
UNLABELLED: Kinsenoside is able to improve bone turnover rate in ovariectomized (OVX) mice. In vitro analysis shows that Kinsenoside antagonizes osteoclast development and bone resorption. INTRODUCTION: Kinsenoside, the main active compound of the traditional Taiwanese herb Anoectochilus formosanus, has an antiinflammatory effect. This study investigates whether Kinsenoside inhibits osteoporosis and osteoclastogenesis. METHODS: OVX mice were used to examine the antiosteoporotic activity of Kinsenoside. The trabecular bone microarchitecture was assessed by microcomputed tomography. In vitro experiments were performed to determine the mechanisms of the antiosteoporotic effects of Kinsenoside. RESULTS: Microcomputed tomography scanning showed that Kinsenoside suppresses bone loss in OVX mice. Kinsenoside decreases plasma CTx concentration. Reverse transcription polymerase chain reaction (RT-PCR) analysis also showed that Kinsenoside reduces the femoral mRNA expression of tartrate-resistant acid phosphatase (TRAP) and matrix metalloproteinase-9 (MMP-9). Kinsenoside inhibits osteoclast formation in bone marrow cells (BMs) and RAW 264.7 cells. Western blot was used to analyze osteoclast-associated signaling pathways in RAW 264.7 cells. Results show that Kinsenoside does not inhibit IKK phosphorylation but suppresses the phosphorylation of IkappaBalpha and p65. Kinsenoside significantly inhibits the RANKL induction of IKK activity. Kinsenoside inhibits the RANKL-triggered nuclear translocations of NF-kappaB and nuclear factor of activated T cells c1 (NFATc1). RT-PCR was used to analyze osteoclast precursor fusion and resorption-associated gene expression in BMs. Kinsenoside inhibits the expression of cathepsin K (CAK), dendritic cell-specific transmembrane protein, MMP-9, and TRAP. CONCLUSIONS: Kinsenoside inhibits osteoclastogenesis from macrophages by attenuating RANKL-induced NF-kappaB and NFATc1 activities, which in turn, prevents bone loss from OVX mice.
The hepatoprotective activity of kinsenoside from Anoectochilus formosanus.[Pubmed:17078107]
Phytother Res. 2007 Jan;21(1):58-61.
Carbon tetrachloride (CCl(4)) causes chronic hepatitis, featuring an increase in hepatic hydroxyproline, spleen weight and serum GPT levels and a decrease in plasma albumin levels. Crude extracts of fresh whole plants of Anoectochilus formosanus showed inhibition of chronic hepatitis induced by CCl(4) in mice. Bioactivity-guided fractionation and spectroscopic analysis revealed that Kinsenoside was the most active compound. In an in vitro study, the LD(50) values for H(2)O(2)-induced cytotoxicity in BALB/c normal liver cells were significantly higher after Kinsenoside pretreatment than after vehicle alone, further confirming that Kinsenoside shows significant antihepatotoxic activity.


