IsovalerylshikoninCAS# 52387-14-1 |
Quality Control & MSDS
Number of papers citing our products

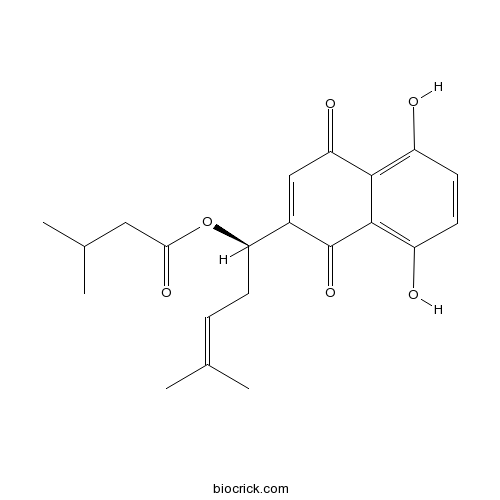
Chemical structure

3D structure
| Cas No. | 52387-14-1 | SDF | Download SDF |
| PubChem ID | 479497 | Appearance | Powder |
| Formula | C21H24O6 | M.Wt | 372.4 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Synonyms | 76549-35-4 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1R)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] 3-methylbutanoate | ||
| SMILES | CC(C)CC(=O)OC(CC=C(C)C)C1=CC(=O)C2=C(C=CC(=C2C1=O)O)O | ||
| Standard InChIKey | UTOUNDHZJFIVPK-QGZVFWFLSA-N | ||
| Standard InChI | InChI=1S/C21H24O6/c1-11(2)5-8-17(27-18(25)9-12(3)4)13-10-16(24)19-14(22)6-7-15(23)20(19)21(13)26/h5-7,10,12,17,22-23H,8-9H2,1-4H3/t17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isovalerylshikonin, a new resistance-modifying agent from Arnebia euchroma, supresses antimicrobial resistance of drug-resistant Staphylococcus aureus; it also has anti-mite activity. Isovalerylshikonin as a candidate of AChE inhibitor, it may prevent apoptotic cell death induced by hydrogen peroxide in human and rat neuronal SH-SY5Y and PC12 cells. |
| Targets | AChR | MAPK | JNK | ERK | c-MYC | AKT | Antifection |
| In vitro | Isovalerylshikonin, a new resistance-modifying agent from Arnebia euchroma, supresses antimicrobial resistance of drug-resistant Staphylococcus aureus.[Pubmed: 30176356 ]Int J Antimicrob Agents. 2019 Jan;53(1):70-73.Antimicrobial resistance is the greatest threat to the treatment of bacterial infectious diseases. The development of resistance-modifying agents (RMAs) represents a promising strategy to mitigate the spread of bacterial antimicrobial resistance.
Identification of Onosma visianii Roots Extract and Purified Shikonin Derivatives as Potential Acaricidal Agents against Tetranychus urticae.[Pubmed: 28621748 ]Molecules. 2017 Jun 16;22(6). pii: E1002.There is an increasing need for the discovery of reliable and eco-friendly pesticides and natural plant-derived products may play a crucial role as source of new active compounds. |
| Kinase Assay | Inhibition of c-MYC with involvement of ERK/JNK/MAPK and AKT pathways as a novel mechanism for shikonin and its derivatives in killing leukemia cells.[Pubmed: 26472107 ]Oncotarget. 2015 Nov 17;6(36):38934-51.Leukemia remains life-threatening despite remarkable advances in chemotherapy. The poor prognosis and drug resistance are challenging treatment. Novel drugs are urgently needed. Shikonin, a natural naphthoquinone, has been previously shown by us to be particularly effective towards various leukemia cell lines compared to solid tumors. However, the underlying mechanisms are still poorly understood.
|
| Cell Research | Acetylshikonin, a Novel AChE Inhibitor, Inhibits Apoptosis via Upregulation of Heme Oxygenase-1 Expression in SH-SY5Y Cells.[Pubmed: 24302971 ]Evid Based Complement Alternat Med. 2013;2013:937370.Acetylcholinesterase inhibitors are prominent alternative in current clinical treatment for AD patients. Therefore, there is a continued need to search for novel AChEIs with good clinical efficacy and less side effects. |

Isovalerylshikonin Dilution Calculator

Isovalerylshikonin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6853 mL | 13.4264 mL | 26.8528 mL | 53.7057 mL | 67.1321 mL |
| 5 mM | 0.5371 mL | 2.6853 mL | 5.3706 mL | 10.7411 mL | 13.4264 mL |
| 10 mM | 0.2685 mL | 1.3426 mL | 2.6853 mL | 5.3706 mL | 6.7132 mL |
| 50 mM | 0.0537 mL | 0.2685 mL | 0.5371 mL | 1.0741 mL | 1.3426 mL |
| 100 mM | 0.0269 mL | 0.1343 mL | 0.2685 mL | 0.5371 mL | 0.6713 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isolappaol A
Catalog No.:BCN8886
CAS No.:131400-96-9
- Pieceid-2''-O-gallate
Catalog No.:BCN8885
CAS No.:105304-51-6
- 3-O-methylellagic acid 4'-O-alpha-L-rhamnopyranoside
Catalog No.:BCN8884
CAS No.:51768-39-9
- Geoside
Catalog No.:BCN8882
CAS No.:585-90-0
- Trachelogenin 4'-O-beta-gentiobioside
Catalog No.:BCN8881
CAS No.:106647-13-6
- Tigloylgomisin O
Catalog No.:BCN8880
CAS No.:130855-74-2
- Gypenoside XIII
Catalog No.:BCN8927
CAS No.:80325-22-0
- Orthosiphol A
Catalog No.:BCN8879
CAS No.:142741-25-1
- Bergaptol-beta-glucopyranoside
Catalog No.:BCN8878
CAS No.:131623-13-7
- Astin C
Catalog No.:BCN8877
CAS No.:148057-23-2
- Neochlorogenic acid methyl ester
Catalog No.:BCN8860
CAS No.:123410-65-1
- Astin B
Catalog No.:BCN8858
CAS No.:151201-76-2
- 2-Methoxyfuranoguaia-9-ene-8-one
Catalog No.:BCN8888
CAS No.:88010-62-2
- Notoptol
Catalog No.:BCN8889
CAS No.:88206-49-9
- Bruceantinol B
Catalog No.:BCN8890
CAS No.:1822332-33-1
- 1,2-Epoxy-10(14)-furanogermacren-6-one
Catalog No.:BCN8891
CAS No.:383368-24-9
- Epischisandrone
Catalog No.:BCN8892
CAS No.:98619-26-2
- Andrographidine A
Catalog No.:BCN8893
CAS No.:113963-37-4
- Quercetin 7-O-(6''-O-malonyl)-beta-D-glucoside
Catalog No.:BCN8894
CAS No.:98767-37-4
- Prionanthoside
Catalog No.:BCN8895
CAS No.:161842-81-5
- N-Methylcorydalmine
Catalog No.:BCN8896
CAS No.:81010-29-9
- 6'-Feruloylnodakenin
Catalog No.:BCN8897
CAS No.:131623-14-8
- 4,4-di(4-hydroxy-3-methoxyphenly)-2,3-dimethylbutanol
Catalog No.:BCN8898
CAS No.:913643-31-9
- [(1(10)E,2R,4R)]-2-Methoxy-8,12-epoxygemacra-1(10),7,11-trien-6-one
Catalog No.:BCN8899
CAS No.:75412-95-2


