IsorhamnetinCAS# 480-19-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 480-19-3 | SDF | Download SDF |
| PubChem ID | 5281654 | Appearance | Yellow powder |
| Formula | C16H12O7 | M.Wt | 316.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 3'-Methylquercetin | ||
| Solubility | DMSO : ≥ 28 mg/mL (88.53 mM) *"≥" means soluble, but saturation unknown. | ||
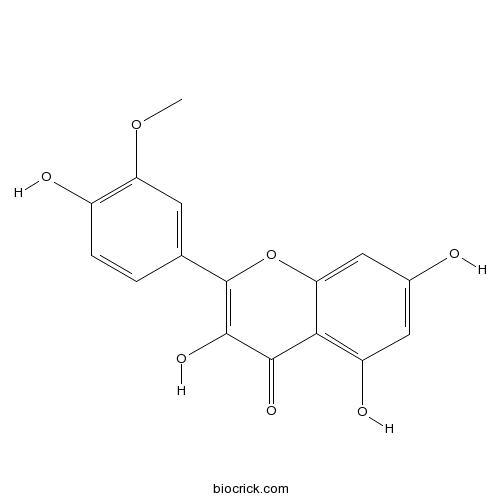
| Chemical Name | 3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=C(C=CC(=C1)C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O | ||
| Standard InChIKey | IZQSVPBOUDKVDZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O7/c1-22-11-4-7(2-3-9(11)18)16-15(21)14(20)13-10(19)5-8(17)6-12(13)23-16/h2-6,17-19,21H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isorhamnetin, a natural flavonol aglycon, is a tyrosinase inhibitor and has anti-adipogenic, cardioprotective, anti-tumor, and antioxidant activities. it inhibits the H(2)O(2)-induced activation of the intrinsic apoptotic pathway via ROS scavenging and ERK inactivation, it inhibits NF-κB signaling. Isorhamnetin prevents angiotensin II (AngII)-induced endothelial dysfunction by inhibiting the overexpression of p47(phox) and the subsequent increases O2-production, resulting in increased nitric oxide bioavailability. |
| Targets | NOS | COX | TNF-α | IL Receptor | NF-kB | p65 | IkB | P-gp | Nrf2 | HO-1 | ROS | ERK | PKC | AMPK | P450 (e.g. CYP17) | PPAR | GSK-3 | Wnt/β-catenin | c-Myc | Caspase | Bcl-2/Bax | IKK |
| In vitro | Isorhamnetin inhibits Prevotella intermedia lipopolysaccharide-induced production of interleukin-6 in murine macrophages via anti-inflammatory heme oxygenase-1 induction and inhibition of nuclear factor-κB and signal transducer and activator of transcrip[Pubmed: 23441850]J Periodontal Res. 2013 Dec;48(6):687-95.Interleukin-6 (IL-6) is a key proinflammatory cytokine that has been considered to be important in the pathogenesis of periodontal disease. Therefore, host-modulatory agents directed at inhibiting IL-6 appear to be beneficial in terms of attenuating periodontal disease progression and potentially improving disease susceptibility. In the current study, we investigated the effect of the flavonoid Isorhamnetin on the production of IL-6 in murine macrophages stimulated with lipopolysaccharide (LPS) from Prevotella intermedia, a pathogen implicated in inflammatory periodontal disease, and its mechanisms of action.
Quercetin and isorhamnetin prevent endothelial dysfunction, superoxide production, and overexpression of p47phox induced by angiotensin II in rat aorta.[Pubmed: 17374653]J Nutr. 2007 Apr;137(4):910-5.The dietary flavonoid quercetin reduces blood pressure and improves endothelial function in several rat models of hypertension.
Isorhamnetin-induced anti-adipogenesis is mediated by stabilization of beta-catenin protein.[Pubmed: 20097210 ]Life Sci. 2010 Mar 13;86(11-12):416-23.Previous studies have shown that Isorhamnetin has anti-adipogenic effects in mouse 3T3-L1 cells. This study was conducted to elucidate the inhibitory mechanisms of Isorhamnetin during adipogenic differentiation of human adipose tissue-derived stem cells (hAMSCs).
|
| Kinase Assay | Effect of Ginkgo biloba extract on procarcinogen-bioactivating human CYP1 enzymes: identification of isorhamnetin, kaempferol, and quercetin as potent inhibitors of CYP1B1.[Pubmed: 16226778 ]Isorhamnetin protects against oxidative stress by activating Nrf2 and inducing the expression of its target genes.[Pubmed: 24211276]Transport characteristics of isorhamnetin across intestinal Caco-2 cell monolayers and the effects of transporters on it.[Pubmed: 24525098]Food Chem Toxicol. 2014 Apr;66:313-20.Flavonoid Isorhamnetin occurs in various plants and herbs, and demonstrates various biological effects in humans.
This work will clarify the Isorhamnetin absorption mechanism using the Caco-2 monolayer cell model.
Toxicol Appl Pharmacol. 2014 Jan 15;274(2):293-301.Isorhamentin is a 3'-O-methylated metabolite of quercetin, and has been reported to have anti-inflammatory and anti-proliferative effects. However, the effects of Isorhamnetin on Nrf2 activation and on the expressions of its downstream genes in hepatocytes have not been elucidated.
Toxicol Appl Pharmacol. 2006 May 15;213(1):18-26.
|
| Cell Research | Isorhamnetin inhibits H₂O₂-induced activation of the intrinsic apoptotic pathway in H9c2 cardiomyocytes through scavenging reactive oxygen species and ERK inactivation.[Pubmed: 21948481 ]J Cell Biochem. 2012 Feb;113(2):473-85.As a traditional Chinese medicine, the sea buckthorn (Hippophae rhamnoides L.) has a long history in the treatment of ischemic heart disease and circulatory disorders. However, the active compounds responsible for and the underlying mechanisms of these effects are not fully understood.
|
| Animal Research | Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway.[Pubmed: 24913217]J Nutr Biochem. 2014 Sep;25(9):923-33.Isorhamnetin is an O-methylated flavonol present in fruit and vegetables. We recently reported the identification of Isorhamnetin as an activator of the human pregnane X receptor (PXR), a known target for abrogating inflammation in inflammatory bowel disease (IBD).
The current study investigated the role of Isorhamnetin as a putative mouse PXR activator in ameliorating chemically induced IBD.
|

Isorhamnetin Dilution Calculator

Isorhamnetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1616 mL | 15.8078 mL | 31.6156 mL | 63.2311 mL | 79.0389 mL |
| 5 mM | 0.6323 mL | 3.1616 mL | 6.3231 mL | 12.6462 mL | 15.8078 mL |
| 10 mM | 0.3162 mL | 1.5808 mL | 3.1616 mL | 6.3231 mL | 7.9039 mL |
| 50 mM | 0.0632 mL | 0.3162 mL | 0.6323 mL | 1.2646 mL | 1.5808 mL |
| 100 mM | 0.0316 mL | 0.1581 mL | 0.3162 mL | 0.6323 mL | 0.7904 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Isorhamnetin is a flavonoid compound extracted from the Chinese herb Hippophae rhamnoides L.. Isorhamnetin suppresses skin cancer through direct inhibition of MEK1 and PI3K.
In Vitro:Isorhamnetin is a plant flavonoid that occurs in fruits and medicinal herbs. Isorhamnetin binds directly to MEK1 in an ATP-noncompetitive manner and to PI3-K in an ATP-competitive manner. In vitro and ex vivo kinase assay data show that Isorhamnetin inhibits the kinase activity of MAP/ERK kinase (MEK) 1 and PI3-K and the inhibition is due to direct binding with Isorhamnetin[1]. Isorhamnetin inhibits the Akt/mTOR and MEK/ERK signaling pathways, and promotes the activity of the mitochondrial apoptosis signaling pathway. The inhibitory effects of Isorhamnetin on breast cancer cells are determined using the CCK-8 method. Isorhamnetin inhibits the proliferation of numerous breast cancer cells (IC50, ~10 µM), including MCF7, T47D, BT474, BT-549, MDA-MB-231 and MDA-MB-468, whereas less inhibitory activity is observed in the MCF10A normal breast epithelial cell line (IC50, 38 µM)[2].
In Vivo:Photographic data shows that Isorhamnetin treatment suppresses tumor development in mice. The average volume of tumors in untreated mice increases over time and reaches a volume of 623 mm3 at 4 weeks post-inoculation; however, at this time, in mice treated with 1 or 5 mg/kg Isorhamnetin, the average tumor volume is only 280 or 198 mm3, respectively. At the end of the study, Isorhamnetin treatment (1 or 5 mg/kg) reduces tumor weight compared with the untreated control group[1].
References:
[1]. Kim JE, et al. Isorhamnetin suppresses skin cancer through direct inhibition of MEK1 and PI3-K. Cancer Prev Res (Phila). 2011 Apr;4(4):582-91.
[2]. Hu S, et al. Isorhamnetin inhibits cell proliferation and induces apoptosis in breast cancer via Akt and mitogen activated protein kinase kinase signaling pathways. Mol Med Rep. 2015 Nov;12(5):6745-51.
- Taxifolin
Catalog No.:BCN5550
CAS No.:480-18-2
- Morin
Catalog No.:BCN1028
CAS No.:480-16-0
- Izalpinine
Catalog No.:BCN3682
CAS No.:480-14-8
- Oroxylin A
Catalog No.:BCN5363
CAS No.:480-11-5
- Astragalin
Catalog No.:BCN5549
CAS No.:480-10-4
- Eleutherol
Catalog No.:BCN8480
CAS No.:480-00-2
- CP-809101
Catalog No.:BCC1498
CAS No.:479683-64-2
- 1-O-galloyl-2-O-cinnamoyl-beta-d-glucose
Catalog No.:BCC3967
CAS No.:
- AUDA
Catalog No.:BCC4023
CAS No.:479413-70-2
- NBQX disodium salt
Catalog No.:BCC6907
CAS No.:479347-86-9
- CNQX disodium salt
Catalog No.:BCC6908
CAS No.:479347-85-8
- TC OT 39
Catalog No.:BCC7958
CAS No.:479232-57-0
- Aromadendrin
Catalog No.:BCN5552
CAS No.:480-20-6
- Orobol
Catalog No.:BCN5553
CAS No.:480-23-9
- Mellein
Catalog No.:BCN4785
CAS No.:480-33-1
- Eugenin
Catalog No.:BCN2921
CAS No.:480-34-2
- Linarin
Catalog No.:BCN5554
CAS No.:480-36-4
- Pinostrobin
Catalog No.:BCN5555
CAS No.:480-37-5
- Pinocembrin
Catalog No.:BCN5556
CAS No.:480-39-7
- Chrysin
Catalog No.:BCN5557
CAS No.:480-40-0
- Naringenin
Catalog No.:BCN5558
CAS No.:480-41-1
- Isosakuranetin
Catalog No.:BCN5559
CAS No.:480-43-3
- Acacetin
Catalog No.:BCN5560
CAS No.:480-44-4
- Hydrangenol
Catalog No.:BCN5561
CAS No.:480-47-7
Quercetin and isorhamnetin prevent endothelial dysfunction, superoxide production, and overexpression of p47phox induced by angiotensin II in rat aorta.[Pubmed:17374653]
J Nutr. 2007 Apr;137(4):910-5.
The dietary flavonoid quercetin reduces blood pressure and improves endothelial function in several rat models of hypertension. We analyzed the effects of quercetin and its methylated metabolite Isorhamnetin on the aortic endothelial dysfunction induced by incubation with angiotensin II (AngII) in vitro for 6 h. AngII diminished the relaxant responses to acetylcholine in phenylephrine-contracted aorta. Coincubation with quercetin or Isorhamnetin, or addition of superoxide (O(2)(-)) dismutase or apocynin to the assay medium, prevented these inhibitory effects. At 6 h, AngII induced a marked increase in O(2)(-) production as measured by dihydroethidium fluorescence, which was prevented by quercetin and Isorhamnetin. AngII also increased the expression of p47(phox), a regulatory subunit of the membrane NADPH oxidase. Immunohistochemical analysis revealed that overexpression of p47(phox) occurred mainly in the medial layer. p47(phox) overexpression was also prevented by quercetin and Isorhamnetin. Taken together, these results show for the first time, to our knowledge, that quercetin and Isorhamnetin prevent AngII-induced endothelial dysfunction by inhibiting the overexpression of p47(phox) and the subsequent increased O(2)(-) production, resulting in increased nitric oxide bioavailability.
Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway.[Pubmed:24913217]
J Nutr Biochem. 2014 Sep;25(9):923-33.
Isorhamnetin is an O-methylated flavonol present in fruit and vegetables. We recently reported the identification of Isorhamnetin as an activator of the human pregnane X receptor (PXR), a known target for abrogating inflammation in inflammatory bowel disease (IBD). The current study investigated the role of Isorhamnetin as a putative mouse PXR activator in ameliorating chemically induced IBD. Using two different models (ulcerative colitis like and Crohn's disease like) of experimental IBD in mice, we demonstrated that Isorhamnetin abrogated inflammation through inhibiting the activity of myeloperoxidase, the levels of TNF-alpha and IL-6, the mRNA expression of proinflammatory mediators (iNOS, ICAM-1, COX2, TNF-alpha, IL-2 and IL-6) and the phosphorylation of IkappaBalpha and NF-kappaB p65. PXR gene overexpression inhibited NF-kappaB luciferase activity, and the inhibition was potentiated by Isorhamnetin treatment. PXR knockdown by siRNA demonstrated the necessity for PXR in Isorhamnetin-mediated up-regulation of xenobiotic metabolism genes. Ligand pocket-filling mutants (S247W/C284W and S247W/C284W/S208W) of human PXR weakened the effect of Isorhamnetin on PXR activation. Molecular docking studies and time-resolved fluorescence resonance energy transfer competitive binding assays confirmed the ligand (Isorhamnetin)-binding affinity. These results clearly demonstrated the ameliorating effect of Isorhamnetin on experimental IBD via PXR-mediated up-regulation of xenobiotic metabolism and down-regulation of NF-kappaB signaling. The novel findings may contribute to the effective utilization of Isorhamnetin or its derivatives as a PXR ligand in the treatment of human IBD.
Effect of Ginkgo biloba extract on procarcinogen-bioactivating human CYP1 enzymes: identification of isorhamnetin, kaempferol, and quercetin as potent inhibitors of CYP1B1.[Pubmed:16226778]
Toxicol Appl Pharmacol. 2006 May 15;213(1):18-26.
In the present study, we investigated the effect of Ginkgo biloba extracts and some of its individual constituents on the catalytic activity of human cytochrome P450 enzymes CYP1B1, CYP1A1, and CYP1A2. G. biloba extract of known abundance of terpene trilactones and flavonol glycosides inhibited 7-ethoxyresorufin O-dealkylation catalyzed by human recombinant CYP1B1, CYP1A1, and CYP1A2, and human liver microsomes, with apparent Ki values of 2 +/- 0.3, 5 +/- 0.5, 16 +/- 1.4, and 39 +/- 1.2 microg/ml (mean +/- SE), respectively. In each case, the mode of inhibition was of the mixed type. Bilobalide, ginkgolides A, B, C, and J, quercetin 3-O-rutinoside, kaempferol 3-O-rutinoside, and isorhamentin 3-O-rutinoside were not responsible for the inhibition of CYP1 enzymes by G. biloba extract, as determined by experiments with these individual chemicals at the levels present in the extract. In contrast, the aglycones of quercetin, kaempferol, and isorhamentin inhibited CYP1B1, CYP1A1, and CYP1A2. Among the three flavonol aglycones, isorhamentin was the most potent in inhibiting CYP1B1 (apparent Ki = 3 +/- 0.1 nM), whereas quercetin was the least potent in inhibiting CYP1A2 (apparent Ki = 418 +/- 50 nM). The mode of inhibition was competitive, noncompetitive, or mixed, depending on the enzyme and the flavonol. G. biloba extract also reduced benzo[a]pyrene hydroxylation, and the effect was greater with CYP1B1 than with CYP1A1 as the catalyst. Overall, our novel findings indicate that G. biloba extract and the flavonol aglycones Isorhamnetin, kaempferol, and quercetin preferentially inhibit the in vitro catalytic activity of human CYP1B1.
Isorhamnetin-induced anti-adipogenesis is mediated by stabilization of beta-catenin protein.[Pubmed:20097210]
Life Sci. 2010 Mar 13;86(11-12):416-23.
AIMS: Previous studies have shown that Isorhamnetin has anti-adipogenic effects in mouse 3T3-L1 cells. This study was conducted to elucidate the inhibitory mechanisms of Isorhamnetin during adipogenic differentiation of human adipose tissue-derived stem cells (hAMSCs). MAIN METHODS: The effect of Isorhamnetin on adipogenic differentiation of hAMSCs was quantified by Oil Red O staining and a triglyceride assay. In addition, real-time PCR and Western blot were used to determine the expression of adipogenesis-related genes. KEY FINDINGS: Isorhamnetin inhibited the adipocyte differentiation of hAMSCs. Additionally, when the effects of Wnt antagonists that promote adipogenesis were evaluated, Isorhamnetin was found to down-regulate the mRNA levels of sFRP1 and Dkk1, but had no effect on the mRNA levels of sFRP2, sFRP3, sFRP4 and Dkk3. Isorhamnetin also inhibited the expression of Wnt receptor and co-receptor genes. Furthermore, Isorhamnetin increased the protein levels of beta-catenin, an effector molecule of Wnt signaling, but had no effect on the mRNA levels of beta-catenin. The phosphorylation level of GSK 3beta was also increased by Isorhamnetin. These results were confirmed by the fact that the expression of c-myc, cyclin D1 and PPARdelta, which are target genes of beta-catenin, was upregulated by Isorhamnetin. Moreover, Isorhamnetin reduced the mRNA expression levels of C/EBPalpha and PPARgamma, which are known to be inhibited by c-myc or by cyclin D1 and PPARdelta, respectively. SIGNIFICANCE: Our results indicate that Isorhamnetin inhibits the adipogenic differentiation of hAMSCs and that its mechanisms are mediated by the stabilization of beta-catenin.
Isorhamnetin inhibits H(2)O(2)-induced activation of the intrinsic apoptotic pathway in H9c2 cardiomyocytes through scavenging reactive oxygen species and ERK inactivation.[Pubmed:21948481]
J Cell Biochem. 2012 Feb;113(2):473-85.
As a traditional Chinese medicine, the sea buckthorn (Hippophae rhamnoides L.) has a long history in the treatment of ischemic heart disease and circulatory disorders. However, the active compounds responsible for and the underlying mechanisms of these effects are not fully understood. In this article, Isorhamnetin pretreatment counteracted H(2)O(2)-induced apoptotic damage in H9c2 cardiomyocytes. Isorhamnetin did not inhibit the death receptor-dependent or extrinsic apoptotic pathways, as characterized by its absence in both caspase-8 inactivation and tBid downregulation along with unchanged Fas and TNFR1 mRNA levels. Instead, Isorhamnetin specifically suppressed the mitochondria-dependent or intrinsic apoptotic pathways, as characterized by inactivation of caspase-9 and -3, maintenance of the mitochondrial membrane potential (DeltaPsim), and regulation of a series of Bcl-2 family genes upstream of DeltaPsim. The anti-apoptotic effects of Isorhamnetin were linked to decreased ROS generation. H(2)O(2) activated ERK and p53, whereas Isorhamnetin inhibited their activation. ERK overexpression overrode the Isorhamnetin-induced inhibition of the intrinsic apoptotic pathway in H9c2 cardiomyocytes, which indicated that an ERK-dependent pathway was involved. Furthermore, N-acetyl cysteine (a potent ROS scavenger) could attenuate the H(2)O(2)-induced apoptosis. However, PD98059 (an ERK-specific inhibitor) could not effectively antagonize ROS generation, which indicates that ROS may be an upstream inducer of ERK. In conclusion, Isorhamnetin inhibits the H(2)O(2)-induced activation of the intrinsic apoptotic pathway via ROS scavenging and ERK inactivation. Therefore, Isorhamnetin is a promising reagent for the treatment of ROS-induced cardiomyopathy.
Isorhamnetin inhibits Prevotella intermedia lipopolysaccharide-induced production of interleukin-6 in murine macrophages via anti-inflammatory heme oxygenase-1 induction and inhibition of nuclear factor-kappaB and signal transducer and activator of transcription 1 activation.[Pubmed:23441850]
J Periodontal Res. 2013 Dec;48(6):687-95.
BACKGROUND AND OBJECTIVE: Interleukin-6 (IL-6) is a key proinflammatory cytokine that has been considered to be important in the pathogenesis of periodontal disease. Therefore, host-modulatory agents directed at inhibiting IL-6 appear to be beneficial in terms of attenuating periodontal disease progression and potentially improving disease susceptibility. In the current study, we investigated the effect of the flavonoid Isorhamnetin on the production of IL-6 in murine macrophages stimulated with lipopolysaccharide (LPS) from Prevotella intermedia, a pathogen implicated in inflammatory periodontal disease, and its mechanisms of action. MATERIAL AND METHODS: Lipopolysaccharide from P. intermedia ATCC 25611 was isolated using the standard hot phenol-water method. Culture supernatants were collected and assayed for IL-6. We used real-time PCR to quantify IL-6 and heme oxygenase-1 (HO-1) mRNA expression. The expression of HO-1 protein and the levels of signaling proteins were monitored using immunoblot analyses. The DNA-binding activity of nuclear factor-kappaB (NF-kappaB) was analyzed using ELISA-based assay kits. RESULTS: Isorhamnetin significantly down-regulated P. intermedia LPS-induced production of IL-6 as well as its mRNA expression in RAW264.7 cells. Isorhamnetin up-regulated the expression of HO-1 at both gene transcription and translation levels in cells stimulated with P. intermedia LPS. In addition, inhibition of HO-1 activity by tin protoporphyrin IX blocked the inhibitory effect of Isorhamnetin on IL-6 production. Isorhamnetin failed to prevent LPS from activating either c-Jun N-terminal kinase or p38 pathways. Isorhamnetin did not inhibit NF-kappaB transcriptional activity at the level of inhibitory kappaB-alpha degradation. Isorhamnetin suppressed NF-kappaB signaling through inhibition of nuclear translocation and DNA binding activity of NF-kappaB p50 subunit and attenuated signal transducer and activator of transcription 1 signaling. CONCLUSION: Although further research is required to clarify the detailed mechanism of action, we propose that Isorhamnetin may contribute to blockade of the host-destructive processes mediated by IL-6 and could be a highly efficient modulator of the host response in the treatment of inflammatory periodontal disease. Further research in animal models of periodontitis is required to better evaluate, the potential of Isorhamnetin as a novel agent for treating periodontal disease.
Isorhamnetin protects against oxidative stress by activating Nrf2 and inducing the expression of its target genes.[Pubmed:24211276]
Toxicol Appl Pharmacol. 2014 Jan 15;274(2):293-301.
Isorhamentin is a 3'-O-methylated metabolite of quercetin, and has been reported to have anti-inflammatory and anti-proliferative effects. However, the effects of Isorhamnetin on Nrf2 activation and on the expressions of its downstream genes in hepatocytes have not been elucidated. Here, we investigated whether Isorhamnetin has the ability to activate Nrf2 and induce phase II antioxidant enzyme expression, and to determine the protective role of Isorhamnetin on oxidative injury in hepatocytes. In HepG2 cells, Isorhamnetin increased the nuclear translocation of Nrf2 in a dose- and time-dependent manner, and consistently, increased antioxidant response element (ARE) reporter gene activity and the protein levels of hemeoxygenase (HO-1) and of glutamate cysteine ligase (GCL), which resulted in intracellular GSH level increases. The specific role of Nrf2 in Isorhamnetin-induced Nrf2 target gene expression was verified using an ARE-deletion mutant plasmid and Nrf2-knockout MEF cells. Deletion of the ARE in the promoter region of the sestrin2 gene, which is recently identified as the Nrf2 target gene by us, abolished the ability of Isorhamnetin to increase luciferase activity. In addition, Nrf2 deficiency completely blocked the ability of Isorhamnetin to induce HO-1 and GCL. Furthermore, Isorhamnetin pretreatment blocked t-BHP-induced ROS production and reversed GSH depletion by t-BHP and consequently, due to reduced ROS levels, decreased t-BHP-induced cell death. In addition Isorhamnetin increased ERK1/2, PKCdelta and AMPK phosphorylation. Finally, we showed that Nrf2 deficiency blocked the ability of Isorhamnetin to protect cells from injury induced by t-BHP. Taken together, our results demonstrate that Isorhamnetin is efficacious in protecting hepatocytes against oxidative stress by Nrf2 activation and in inducing the expressions of its downstream genes.
Transport characteristics of isorhamnetin across intestinal Caco-2 cell monolayers and the effects of transporters on it.[Pubmed:24525098]
Food Chem Toxicol. 2014 Apr;66:313-20.
Flavonoid Isorhamnetin occurs in various plants and herbs, and demonstrates various biological effects in humans. This work will clarify the Isorhamnetin absorption mechanism using the Caco-2 monolayer cell model. The Isorhamnetin transport characteristics at different concentrations, pHs, temperatures, tight junctions and potential transporters were systemically investigated. Isorhamnetin was poorly absorbed by both passive diffusion and active transport mechanisms. Both trans- and paracellular pathways were involved during Isorhamnetin transport. Active transport under an ATP-dependent transport mechanism was mediated by the organic anion transporting peptide (OATP); Isorhamnetin's permeability from the apical to the basolateral side significantly decreased after estrone-3-sulfate was added (p<0.01). Efflux transporters, P-glycoproteins (P-gp), breast cancer resistance proteins (BCRP) and multidrug resistance proteins (MRPs) participated in the Isorhamnetin transport process. Among them, the MRPs (especially MRP2) were the main efflux transporters for Isorhamnetin; transport from the apical to the basolateral side increased 10.8-fold after adding an MRP inhibitor (MK571). This study details Isorhamnetin's cellular transport and elaborates Isorhamnetin's absorption mechanisms to provide a foundation for further studies.
In vitro anti-tumor activity of isorhamnetin isolated from Hippophae rhamnoides L. against BEL-7402 cells.[Pubmed:16765054]
Pharmacol Res. 2006 Sep;54(3):186-94.
Isorhamnetin, a flavonol aglycone, isolated from the traditional Chinese medicine Hippophae rhamnoides L., was investigated in its cytotoxicity and its influence on human hepatocellular carcinoma cells (BEL-7402). The cytotoxic effects of Isorhamnetin showed dose- and time-dependency against BEL-7402 cells, with IC(50) equal to 74.4+/-1.13 microg ml(-1) after treatment with Isorhamnetin for 72 h. Cytotoxicity of the flavonols on tumor cells depends on cellular accumulation of the drugs. The amount of Isorhamnetin accumulated in BEL-7402 cells was assayed by high-performance liquid chromatography (HPLC) and showed that Isorhamnetin could permeate the cell membrane into the cell. Staining with Hoechst 33258 showed fragmentation and condensation of chromatin in the cell treated with 50 microg ml(-1) Isorhamnetin for 48 h. Flow cytometric analysis was performed to determine hypodiploid cells. The results of flow cytometry assay indicated that the percentage of hypodiploid BEL-7402 cells were 13.77+/-1.05% after 48 h treatment with 50 microg ml(-1) Isorhamnetin. The treatment resulted in the appearance of a hypodiploid peak (sub-G(0)/G(1) peak), probably due to the presence of cells in apoptosis and apoptotic bodies with DNA content less than 2n. To our knowledge, this is the first report against human hepatocellular carcinoma cells (BEL-7402) of Isorhamnetin.


