IsodihydroauroglaucinCAS# 74886-31-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 74886-31-0 | SDF | Download SDF |
| PubChem ID | 14355115 | Appearance | Yellow powder |
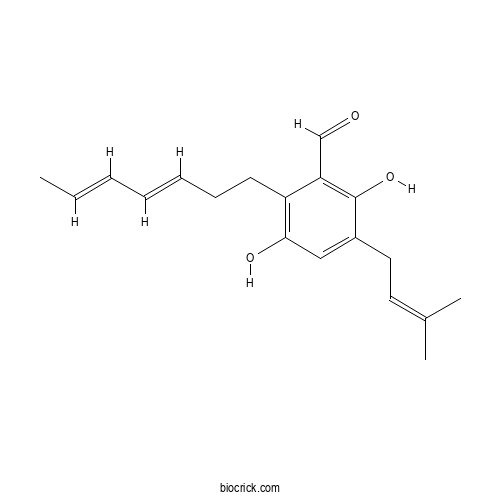
| Formula | C19H24O3 | M.Wt | 300.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-[(3E,5E)-hepta-3,5-dienyl]-3,6-dihydroxy-5-(3-methylbut-2-enyl)benzaldehyde | ||
| SMILES | CC=CC=CCCC1=C(C=C(C(=C1C=O)O)CC=C(C)C)O | ||
| Standard InChIKey | ZNSOEVHEUKFQSM-YTXTXJHMSA-N | ||
| Standard InChI | InChI=1S/C19H24O3/c1-4-5-6-7-8-9-16-17(13-20)19(22)15(12-18(16)21)11-10-14(2)3/h4-7,10,12-13,21-22H,8-9,11H2,1-3H3/b5-4+,7-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isodihydroauroglaucin Dilution Calculator

Isodihydroauroglaucin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3289 mL | 16.6445 mL | 33.2889 mL | 66.5779 mL | 83.2224 mL |
| 5 mM | 0.6658 mL | 3.3289 mL | 6.6578 mL | 13.3156 mL | 16.6445 mL |
| 10 mM | 0.3329 mL | 1.6644 mL | 3.3289 mL | 6.6578 mL | 8.3222 mL |
| 50 mM | 0.0666 mL | 0.3329 mL | 0.6658 mL | 1.3316 mL | 1.6644 mL |
| 100 mM | 0.0333 mL | 0.1664 mL | 0.3329 mL | 0.6658 mL | 0.8322 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Hydroxyphenylpyruvic acid
Catalog No.:BCX0519
CAS No.:156-39-8
- Sanggenon F
Catalog No.:BCX0518
CAS No.:85889-03-8
- Tetrahydroauroglaucin
Catalog No.:BCX0517
CAS No.:40434-07-9
- 5,4'-Dihydroxy-6,7,8,3'-tetramethoxyflavone
Catalog No.:BCX0516
CAS No.:16520-78-8
- 5,7-Dihydroxy-3,8,3',4'-tetramethoxyflavone
Catalog No.:BCX0515
CAS No.:42923-42-2
- Xanthomicrol
Catalog No.:BCX0514
CAS No.:16545-23-6
- N-Acetyl-D-mannosamine
Catalog No.:BCX0513
CAS No.:7772-94-3
- N-acetyl-D-galactosamine
Catalog No.:BCX0512
CAS No.:1811-31-0
- D-Galactosamine hydrochloride
Catalog No.:BCX0511
CAS No.:1772-03-8
- D-Mannosamine hydrochloride
Catalog No.:BCX0510
CAS No.:5505-63-5
- Moracin N
Catalog No.:BCX0509
CAS No.:135248-05-4
- (S)-5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-7-phenylheptan-3-one
Catalog No.:BCX0508
CAS No.:1220110-76-8
- Demethoxysudachitin
Catalog No.:BCX0521
CAS No.:4323-80-2
- Oxyphyllenodiol B
Catalog No.:BCX0522
CAS No.:363610-32-6
- Labda-12E,14-dien-16,15-olid-17-oic acid
Catalog No.:BCX0523
CAS No.:1855905-16-6
- Butyl rosmarinate
Catalog No.:BCX0524
CAS No.:222713-83-9
- Dihydroauroglaucin
Catalog No.:BCX0525
CAS No.:77102-91-1
- (E)-5-Hydroxy-6-isoprenyl-2-(pent-1-en-1-yl)benzofuran-4-carbaldehyde
Catalog No.:BCX0526
CAS No.:916602-30-7
- 5-(1-Hydroxypropan-2-yl)-2-methylphenol
Catalog No.:BCX0527
CAS No.:111044-80-5
- 5,7,4'-Trihydroxy-3,8-dimethoxyflavone
Catalog No.:BCX0528
CAS No.:14965-09-4
- 4-Hydroxythonningianin B
Catalog No.:BCX0529
CAS No.:2329726-95-4
- 2-Hydroxymethyl-5-isopropylphenol
Catalog No.:BCX0530
CAS No.:111044-81-6
- Salcolin A
Catalog No.:BCX0531
CAS No.:1977557-69-9
- 1β,8α-Dihydroxyeremophila-7(11),9-dien-12,8-olide
Catalog No.:BCX0532
CAS No.:849700-44-3
Characterization of Hypolipidemic Phenol Analogues from Fermented Tea by Eurotium cristatum.[Pubmed:36613264]
Foods. 2022 Dec 22;12(1):49.
Fuzhuan brick tea (FBT), a type of black tea, is a traditional beverage in China, especially popular among frontier ethnic groups. FBT is well-known for its health benefits, such as hypoglycemic, anti-hypertensive, anti-inflammatory, diuretic, and detoxification effects. Nevertheless, the underlying mechanisms on the molecular level are still elusive and the key compounds responsible for the health benefits are unidentified. Previous studies have mainly focused on functional studies of the water extract. However, FBT is typically cooked with butter or milk. Therefore, we hypothesized that some lipophilic components in FBT, which can be absorbed through the co-consumption of butter or milk, may play an important role in the health benefits. The present study aimed to investigate whether the liposoluble extract of FBT alleviates symptoms related to metabolic diseases and to identify the active compounds involved. By comparing the high-performance liquid chromatography (HPLC) profiles of water, milk and hexane extract, some low polarity peaks were observed in the milk and hexane extracts. Furthermore, the hexane extract treatment alleviated body weight gain, serum total cholesterol and triglyceride levels, and inhibited the accumulation of hepatic fat granules in a high-fat diet (HFD)-induced C57BL/6N mouse model. In order to identify the key functional lipophilic compounds in FBT, the hexane extract of FBT was subjected to chemical characterization. Four phenol analogs were characterized, namely, Isodihydroauroglaucin (1), dihydroauroglaucin (2), tetrahydroauroglaucin (3), and flavoglaucin (4). Compounds 1 and 4 reduced the levels of total cholesterol and triglyceride in vivo. Both compounds also inhibited the high-fat diet-induced body weight gain and accumulation of fat granules in the liver of C57BL/6N mice. Isodihydroauroglaucin and flavoglaucin have therefore been identified as bioactive ingredients that contribute to the health benefits of FBT.
Anti-aging efficacy of solid-state fermented ginseng with Aspergillus cristatus and its active metabolites.[Pubmed:36250021]
Front Mol Biosci. 2022 Sep 29;9:984307.
Aspergillus cristatus is a beneficial fungus of microbial fermented teas such as China's Fuzhuan brick tea and Pu-erh tea, and is commonly called golden flower fungus (GFF) because its cleistothecium has a yellow millet or sand grain shape. Since natural materials fermented with GFF exhibit various physiological activities, a new active cosmeceutical ingredient was developed by solid-state fermentation of ginseng, a famous active material for healthy skin, with GFF. The extract of solid-state fermented ginseng with GFF (GFFG) exhibited potent anti-aging efficacy on the skin such as the increase of hyaluronic acid synthesis, aquaporin expression, and mRNA level of filaggrin in HaCaT keratinocyte. GFFG also inhibited the expression of MMP-1 increased by TNF-alpha in human dermal fibroblast. Sophisticated chromatographic and spectroscopic studies have elucidated Isodihydroauroglaucin and flavoglaucin as the metabolites which were not present in ginseng extract nor GFF extract alone. Bioassay of these metabolites revealed that these compounds were part of active principles of GFFG. These results suggest that GFFG would be a potential active ingredient in anti-aging cosmeceutical products.
Separation of six antioxidants from Hypsizygus marmoreus by high-speed countercurrent chromatography utilizing an approach based upon the polarity parameter model.[Pubmed:33160255]
J Chromatogr A. 2020 Dec 6;1633:461650.
For a successful high-speed countercurrent chromatography (HSCCC) separation of multiple components, the suitable solvent system selection is a critical operation. Despite the difference in separation mechanism between HSCCC and HPLC, K values of compounds in the solvent system and the retention factor of compounds in HPLC were connected with polarity, and the polarity mainly depended on the structure and chemical properties of compounds. On the basis of the concept of "like dissolves like", the average polarity of the solvent system is equal to the average polarity of multiple components at the "sweet point" log K = 0. The result of theoretical deduction showed a negative linear correlation between the polarity of each component and the logarithm of the gradient range of the organic phase. Therefore, the average polarity of unknown multiple components could be obtained through the polarity parameter model established in the HPLC analysis. The suitable solvent system composed of n-hexane-ethyl acetate-methanol-water (8:3:8:1, v/v) was selected through the average polarity of multi-components combining K values of the enriched samples. In the context of bioassay, an efficient HSCCC separation procedure was established, and two groups of analogue benzaldehyde derivatives (four main antioxidants, namely, Isodihydroauroglaucin, isoaspergin, isotetrahydro-auroglaucin, and flavoglaucin; two minor antioxidants, namely, 6'-oxo-chaetopyranin and chaetopyranin) were obtained from Hypsizygus marmoreus. The predicted polarity values of multi-components were sufficient to meet the HSCCC experimental requirements. The HPLC analysis of reference compounds and multi-components showed a significant consistency with different chromatographic columns. Therefore, the polarity parameter model established in the HPLC analysis was a simple, rapid, and helpful tool for looking an appropriate solvent system, which was a forwarding step for the HSCCC separation of multiple components.
Marine Fungi from the Sponge Grantia compressa: Biodiversity, Chemodiversity, and Biotechnological Potential.[Pubmed:30978942]
Mar Drugs. 2019 Apr 11;17(4):220.
The emergence of antibiotic resistance and viruses with high epidemic potential made unexplored marine environments an appealing target source for new metabolites. Marine fungi represent one of the most suitable sources for the discovery of new compounds. Thus, the aim of this work was (i) to isolate and identify fungi associated with the Atlantic sponge Grantia compressa; (ii) to study the fungal metabolites by applying the OSMAC approach (one strain; many compounds); (iii) to test fungal compounds for their antimicrobial activities. Twenty-one fungal strains (17 taxa) were isolated from G. compressa. The OSMAC approach revealed an astonishing metabolic diversity in the marine fungus Eurotium chevalieri MUT 2316, from which 10 compounds were extracted, isolated, and characterized. All metabolites were tested against viruses and bacteria (reference and multidrug-resistant strains). Dihydroauroglaucin completely inhibited the replication of influenza A virus; as for herpes simplex virus 1, total inhibition of replication was observed for both physcion and neoechinulin D. Six out of 10 compounds were active against Gram-positive bacteria with Isodihydroauroglaucin being the most promising compound (minimal inhibitory concentration (MIC) 4-64 microg/mL) with bactericidal activity. Overall, G. compressa proved to be an outstanding source of fungal diversity. Marine fungi were capable of producing different metabolites; in particular, the compounds isolated from E. chevalieri showed promising bioactivity against well-known and emerging pathogens.
Evaluation of flavoglaucin, its derivatives and pyranonigrins produced by molds used in fermented foods for inhibiting tumor promotion.[Pubmed:20460698]
Biosci Biotechnol Biochem. 2010;74(5):1120-2.
Flavoglaucin, its derivatives, and pyranonigrins, which are antioxidants produced by the molds used in fermented foods, were examined for their inhibition of tumor promotion by the Epstein-Barr virus early antigen activation test. Flavoglaucin and its derivatives exhibited high activity. Flavoglaucin and such a derivative as Isodihydroauroglaucin inhibited mouse skin tumor promotion in a two-stage carcinogenesis test and appear to be antitumor promoters.
Antioxidants produced by Eurotium herbariorum of filamentous fungi used for the manufacture of karebushi, dried bonito (Katsuobushi).[Pubmed:19502740]
Biosci Biotechnol Biochem. 2009 Jun;73(6):1323-7.
Extracts prepared by culturing ten filamentous fungi from Aspergillus and Eurotium species isolated from dried bonito (katsuobushi) were examined for 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging capacity. The extracts prepared by culturing E. herbariorum NE-1 and NE-4, which are used in the molding process for the manufacture of karebushi (a kind of katsuobushi), were shown to have higher activity than the others. Five antioxidants were isolated from the extracts and identified as Isodihydroauroglaucin (IDAG), auroglaucin (AG), dihydroauroglaucin (DAG), tetrahydroauroglaucin (TAG), and flavoglaucin (FG) by (1)H-NMR, (13)C-NMR, and EI-MS analyses. Compared with alpha-tocopherol, the isolated antioxidants exhibited high antioxidative activity for the radical scavenging capacity of DPPH and superoxide, but low activity for inhibiting the autoxidation of docosahexaenoic acid (DHA). The isolated antioxidants were produced by the Eurotium species, but not by the Aspergillus species. DAG and TAG exhibited higher radical scavenging capacity than the other antioxidants and were abundantly contained in the extracts of E. herbariorum NE-1 and NE-4.
A new radical scavenging anthracene glycoside, asperflavin ribofuranoside, and polyketides from a marine isolate of the fungus microsporum.[Pubmed:16755063]
Chem Pharm Bull (Tokyo). 2006 Jun;54(6):882-3.
A new anthracene glycoside, asperflavin ribofuranoside (1), and the previously described polyketides, flavoglaucin (2), Isodihydroauroglaucin (3), and citrinin (4) have been isolated from the marine-derived fungus Microsporum sp. The structure and absolute stereochemistry of a new compound (1) was assigned on the basis of physicochemical data. Compounds 1-3 exhibited a significant radical scavenging activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH) with IC(50) values of 14.2, 11.3, and 11.5 microM, respectively, which are more potent than the positive control, ascorbic acid (IC(50), 20 microM). Compound 1 also showed a moderate antibacterial activity against the methicillin-resistant and multidrug-resistant Staphylococcus aureus (MRSA and MDRSA) with MIC value of 50 microg/ml.


