Iristectorigenin ACAS# 86849-77-6 |
- Iristectorigenin B
Catalog No.:BCN8391
CAS No.:39012-01-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 86849-77-6 | SDF | Download SDF |
| PubChem ID | 5488781 | Appearance | White crystalline powder |
| Formula | C17H14O7 | M.Wt | 330.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Iristectorigenin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
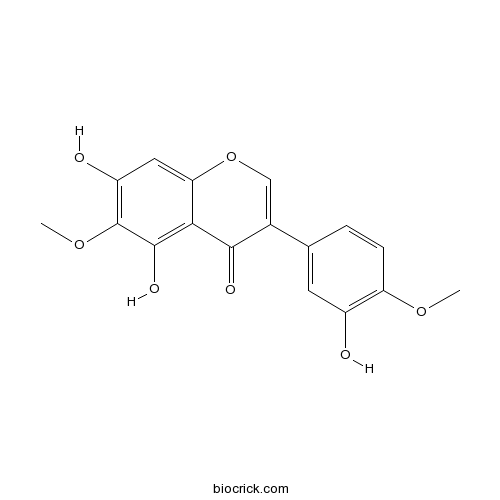
| Chemical Name | 5,7-dihydroxy-3-(3-hydroxy-4-methoxyphenyl)-6-methoxychromen-4-one | ||
| SMILES | COC1=C(C=C(C=C1)C2=COC3=CC(=C(C(=C3C2=O)O)OC)O)O | ||
| Standard InChIKey | WRZOUWHPDDOJNR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14O7/c1-22-12-4-3-8(5-10(12)18)9-7-24-13-6-11(19)17(23-2)16(21)14(13)15(9)20/h3-7,18-19,21H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Iristectorigenin A Dilution Calculator

Iristectorigenin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0276 mL | 15.1378 mL | 30.2755 mL | 60.551 mL | 75.6888 mL |
| 5 mM | 0.6055 mL | 3.0276 mL | 6.0551 mL | 12.1102 mL | 15.1378 mL |
| 10 mM | 0.3028 mL | 1.5138 mL | 3.0276 mL | 6.0551 mL | 7.5689 mL |
| 50 mM | 0.0606 mL | 0.3028 mL | 0.6055 mL | 1.211 mL | 1.5138 mL |
| 100 mM | 0.0303 mL | 0.1514 mL | 0.3028 mL | 0.6055 mL | 0.7569 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nuezhenide
Catalog No.:BCN5444
CAS No.:39011-92-2
- Oxypaeoniflorin
Catalog No.:BCN6346
CAS No.:39011-91-1
- Albiflorin
Catalog No.:BCN1264
CAS No.:39011-90-0
- 2-Acetyl-6-methoxynaphthalene
Catalog No.:BCC8514
CAS No.:3900-45-6
- Gelsevirine
Catalog No.:BCN5443
CAS No.:38990-03-3
- (S)-CPW 399
Catalog No.:BCC7106
CAS No.:389888-02-2
- α-Tocopherol phosphate
Catalog No.:BCC5420
CAS No.:38976-17-9
- Ophiopogonin B
Catalog No.:BCN5378
CAS No.:38971-41-4
- 2'-O-Methylperlatolic acid
Catalog No.:BCN5442
CAS No.:38968-07-9
- Eriodictyol-7-O-glucoside
Catalog No.:BCN4743
CAS No.:38965-51-4
- N-Acetylnorloline
Catalog No.:BCN2005
CAS No.:38964-35-1
- Raspberry ketone glucoside
Catalog No.:BCC8244
CAS No.:38963-94-9
- Epimedoside A
Catalog No.:BCN2886
CAS No.:39012-04-9
- Picroside II
Catalog No.:BCN6323
CAS No.:39012-20-9
- (E)-2-(3-(3,4-dimethoxyphenyl)acryloyloxy)succinic acid
Catalog No.:BCN8545
CAS No.:39015-77-5
- Wilsonine
Catalog No.:BCN5445
CAS No.:39024-12-9
- 3-Epiwilsonine
Catalog No.:BCN5446
CAS No.:39024-15-2
- Z-Guggulsterone
Catalog No.:BCC7712
CAS No.:39025-23-5
- Guggulsterone E
Catalog No.:BCC8181
CAS No.:39025-24-6
- 9-Methoxycamptothecine
Catalog No.:BCN1219
CAS No.:39026-92-1
- Buxbodine B
Catalog No.:BCN5447
CAS No.:390362-51-3
- Buxbodine D
Catalog No.:BCN5448
CAS No.:390362-53-5
- GS 39783
Catalog No.:BCC7233
CAS No.:39069-52-8
- Ingenol 20-palmitate
Catalog No.:BCN7678
CAS No.:39071-33-5
Dynamic changes of flavonoids contents in the different parts of rhizome of Belamcanda chinensis during the thermal drying process.[Pubmed:25036154]
Molecules. 2014 Jul 17;19(7):10440-54.
The dried rhizome of Belamcanda. chinensis (L.) DC. is an important traditional Chinese medicine. Previous chemical and pharmacological investigations indicated that flavonoids may be responsible for the bioactivity of the herb. In this paper, the effects on the contents of twelve flavonoids in the three subunit parts of the rhizome of B. chinensis during the thermal drying process under treatment temperatures ranging from 40 degrees C to 120 degrees C at 10 degrees C intervals were investigated. The results showed that the content of most of the individual flavonoids except that of tectorigenin in the fresh eldest parts of the rhizome that originate directly from the seedling was higher than those of the other junior parts. The change trends of flavonoids contents were similar for three subunit parts of the rhizome during the drying process under the same treatment temperature. Most of the individual flavonoid contents in the rhizome increased in the early stages of the drying processes and decreased as the process was prolonged. The durations required to reaching the points of the maximal amounts of flavonoids revealed a significant negative correlation with the temperature. The variation of the content of mangiferin, Iristectorigenin A, irigenin, irilone and dichotomitin was positively correlated with irisflorentin that is the chemical marker used for the quality control of this herb. Taking into account of the production effectiveness and flavonoid yields, the appropriate drying temperature for this herb was suggested to be 100 degrees C.
Polyphenolic compounds from callus cultures of Iris pseudacorus.[Pubmed:24354190]
Nat Prod Commun. 2013 Oct;8(10):1419-20.
A callus culture of Iris pseudacorus L. (Iridaceae) was established from plant leaves using a modified Murashige and Skoog medium. A derivative of cinnamic acid (lavandoside) (1), a neolignan (dehydrodiconiferyl alcohol-4-O-beta-D-glucopyranoside) (2) as well as three isoflavonoids, tectoridin (3), tectorigenin (4), and Iristectorigenin A (5) were isolated from the callus culture. Under normal conditions, the calli accumulated 0.4% DW of polyphenols. The addition of phenylalanine to a concentration of 1 mM resulted in a 1.5-fold increase in isoflavonoid production, allowing the accumulation of 0.69% of polyphenols in the callus dry weight. Tectorigenin, a promising chemotherapeutic and chemopreventive agent for the treatment of carcinomas, was produced in I. pseudacorus calli in high quantities (0.3% DW).
Ionic-liquid-based ultrasound-assisted extraction of isoflavones from Belamcanda chinensis and subsequent screening and isolation of potential alpha-glucosidase inhibitors by ultrafiltration and semipreparative high-performance liquid chromatography.[Pubmed:28444982]
J Sep Sci. 2017 Jun;40(12):2565-2574.
The separation of a compound of interest from its structurally similar homologues to produce high-purity natural products is a challenging problem. This work proposes a novel method for the separation of Iristectorigenin A from its structurally similar homologues by ionic-liquid-based ultrasound-assisted extraction and the subsequent screening and isolation of potential alpha-glucosidase inhibitors via ultrafiltration and semipreparative high-performance liquid chromatography. Ionic-liquid-based ultrasound-assisted extraction was successfully applied to the extraction of tectorigenin, Iristectorigenin A, irigenin, and irisflorentin from Belamcanda chinensis. The optimum conditions for the efficient extraction of isoflavones were determined as 1.0 M 1-ethyl-3-methylimidazolium tetrafluoroborate with extraction time of 30 min and a solvent to solid ratio of 30 mL/g. Ultrafiltration with liquid chromatography and mass spectrometry was applied to screen and identify alpha-glucosidase inhibitors from B. chinensis, followed by the application of semipreparative high-performance liquid chromatography to separate and isolate the active constituents. Four major compounds including tectorigenin, Iristectorigenin A, irigenin, and irisflorentin were screened and identified as alpha-glucosidase inhibitors, and then the four active compounds abovementioned were subsequently isolated by semipreparative high-performance liquid chromatography (99.89, 88.97, 99.79, and 99.97% purity, respectively). The results demonstrate that ionic liquid extraction can be successfully applied to the extraction of isoflavones from B. chinensis.
Phenolic metabolite profiles and antioxidants assay of three Iridaceae medicinal plants for traditional Chinese medicine "She-gan" by on-line HPLC-DAD coupled with chemiluminescence (CL) and ESI-Q-TOF-MS/MS.[Pubmed:25106824]
J Pharm Biomed Anal. 2014 Sep;98:40-51.
An on-line analysis method by HPLC-DAD coupled with chemiluminescence (CL) and ESI-Q-TOF-MS/MS was established for simultaneous detection and identification of antioxidants in three original plants of traditional Chinese medicine "She-gan". Two new isoflavonoid glycosides, along with 48 known compounds, including isoflavonoid glycosides and their aglycones, xanthones, flavones and other phenolic compounds, were identified or tentatively identified from the rhizomes of three Iridaceae plants, namely, Belamcanda chinensis, Iris tectorum and Iris dichotoma, which were used as "She-gan" in China. Among those compounds, isoflavone glycosides of Iristectorigenin A and its isomers exhibited obviously inhibit CL, which suggested their strong free radical scavenging activity. The chemometric methods dealing with the data gained by chromatographic and antioxidant activity profiles exhibited the "similarities" and "differences" of chemical constituents and antioxidant activities for three studied Iridaceae species. The results indicated that the established method might provide for qualitative and quantitative evaluation of the herbal medicines.
Methyl caffeate and some plant constituents inhibit age-related inflammation: effects on senescence-associated secretory phenotype (SASP) formation.[Pubmed:28299617]
Arch Pharm Res. 2017 Apr;40(4):524-535.
During aging, cells secrete molecules called senescence-associated secretory phenotype (SASP). They constitute chronic low-grade inflammation environment to adjacent cells and tissues. In order to find inhibiting agents of SASP formation, 113 plant constituents were incubated with BJ fibroblasts for 6 days after treatment with bleomycin. Several plant constituents showed considerable inhibition of IL-6 production, a representative SASP marker. These plant constituents included anthraquinones such as aurantio-obtusin, flavonoids including astragalin, Iristectorigenin A, iristectorigenin B, linarin, lignans including lariciresinol 9-O-glucoside and eleutheroside E, phenylpropanoids such as caffeic acid and methyl caffeate, steroid (ophiopogonin), and others like centauroside, rhoifolin and scoparone. In particular, methyl caffeate down-regulated SASP factors such as IL-1alpha, IL-1beta, IL-6, IL-8, GM-CSF, CXCL1, MCP-2, and MMP-3. Inhibition of these SASP mRNA expression levels also coincided with the reduction of IkappaBzeta expression and NF-kappaB p65 activation without affecting the expression levels of senescence markers, p21 or pRb. Taken together, the present study demonstrated that methyl caffeate might be a specific and strong inhibitor of SASP production without affecting the aging process. Its action mechanisms involve the reduction of IkappaBzeta expression and NF-kappaB p65 activation. Therefore, this compound might be effective in alleviating chronic low-grade inflammation linked to age-related degenerative disorders.
Hepatoprotective activity of LC-ESI-MS standardized Iris spuria rhizome extract on its main bioactive constituents.[Pubmed:24877715]
Phytomedicine. 2014 Sep 15;21(10):1202-7.
The study was designed to evaluate the hepatoprotective activity of Iris spuria against paracetamol induced toxicity at two different doses 100 and 200 mg/kg. The extract showed significant protective activity (p>0.01) at both the doses in dose dependent manner. Administration of the plant extract restored the paracetamol induced elevated levels of serum marker and distorted hepatic tissue architecture. The lipid peroxides (LPO) and glutathione (GSH) levels were also restored towards normal in liver tissue significantly. The main chemical constituents of the extract identified by the liquid chromatography-tandem mass spectrometry (LC-ESI-MSMS) were found to be flavones and isoflavonoids. Tectoridin and Iristectorigenin A were the principal compounds present in the methanolic extract of Iris spuria.


