Irinotecan hydrochlorideDNA topoisomerase I inhibitor; antitumor CAS# 100286-90-6 |
Quality Control & MSDS
Number of papers citing our products

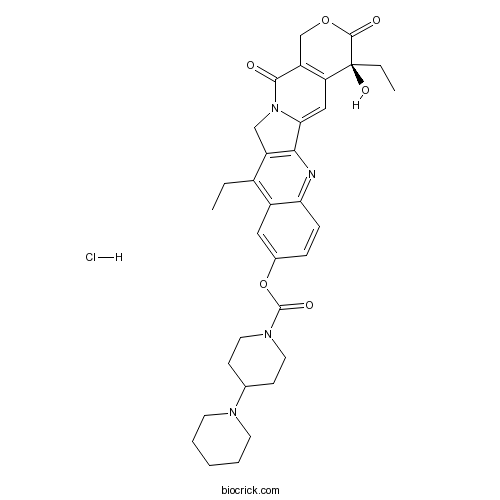
Chemical structure

3D structure
| Cas No. | 100286-90-6 | SDF | Download SDF |
| PubChem ID | 74990 | Appearance | Powder |
| Formula | C33H39ClN4O6 | M.Wt | 623.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Irinotecan, Camptothecin 11 | ||
| Solubility | DMSO : ≥ 51 mg/mL (81.84 mM) H2O : 3.33 mg/mL (5.34 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| SMILES | CCC1=C2C=C(C=CC2=NC3=C1CN4C3=CC5=C(C4=O)COC(=O)C5(CC)O)OC(=O)N6CCC(CC6)N7CCCCC7.Cl | ||
| Standard InChIKey | GURKHSYORGJETM-WAQYZQTGSA-N | ||
| Standard InChI | InChI=1S/C33H38N4O6.ClH/c1-3-22-23-16-21(43-32(40)36-14-10-20(11-15-36)35-12-6-5-7-13-35)8-9-27(23)34-29-24(22)18-37-28(29)17-26-25(30(37)38)19-42-31(39)33(26,41)4-2;/h8-9,16-17,20,41H,3-7,10-15,18-19H2,1-2H3;1H/t33-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Irinotecan hydrochloride is a water soluble topoisomerase I inhibitor mainly used to treat colon cancer and rectal cancer. |
| Targets | Topoisomerase |
| In vitro | Activity of CPT-11 (irinotecan hydrochloride), a topoisomerase I inhibitor, against human tumor colony-forming units.[Pubmed: 8049503]Anticancer Drugs. 1994 Apr;5(2):202-6.CPT-11 (Irinotecan hydrochloride, 7-ethyl-10-[4-(piperidino)-1-piperidino] carbonyloxy-camptothecin) is a semisynthetic camptothecin derivative developed in Japan. |
| In vivo | Inhibition of intestinal microflora beta-glucuronidase modifies the distribution of the active metabolite of the antitumor agent, irinotecan hydrochloride (CPT-11) in rats.[Pubmed: 9744772]Cancer Chemother Pharmacol. 1998;42(4):280-6.SN-38, a metabolite of Irinotecan hydrochloride (CPT-11), is considered to play a key role in the development of diarrhea as well as in the antitumor activity of CPT-11. We have previously found that the inhibition of beta-glucuronidase, which hydrolyzes detoxified SN-38 (SN-38 glucuronide) to reform SN-38, in the lumen by eliminating the intestinal microflora with antibiotics, markedly ameliorates the intestinal toxicity of CPT-11 in rats. In this study we compared the disposition of CPT-11 and its metabolites in rats treated with and without antibiotics.
|

Irinotecan hydrochloride Dilution Calculator

Irinotecan hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6046 mL | 8.0231 mL | 16.0462 mL | 32.0924 mL | 40.1155 mL |
| 5 mM | 0.3209 mL | 1.6046 mL | 3.2092 mL | 6.4185 mL | 8.0231 mL |
| 10 mM | 0.1605 mL | 0.8023 mL | 1.6046 mL | 3.2092 mL | 4.0116 mL |
| 50 mM | 0.0321 mL | 0.1605 mL | 0.3209 mL | 0.6418 mL | 0.8023 mL |
| 100 mM | 0.016 mL | 0.0802 mL | 0.1605 mL | 0.3209 mL | 0.4012 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Irinotecan hydrochloride is a water soluble topoisomerase I inhibitor with antitumor activity.
In Vitro:Irinotecan hydrochloride is a topoisomerase I inhibitor. Irinotecan inhibits the growth of LoVo and HT-29 cells, with IC50s of 15.8 ± 5.1 and 5.17 ± 1.4 μM, respectively, and induces similar amounts of cleavable complexes in both in LoVo and HT-29 cells[2]. Irinotecan suppresses the proliferation of human umbilical vein endothelial cells (HUVEC), with an IC50 of 1.3 μM[3].
In Vivo:Irinotecan (CPT-11, 5 mg/kg) significantly inhibits the growth of tumors by intratumoral injection daily for 5 days, on two consecutive weeks in rats, and such effects also occur via continuous intraperitoneal infusion by osmotic minipump into mice. However, Irinotecan (10 mg/kg) shows no effect on the growth of tumor by i.p[1]. Irinotecan (CPT-11, 100-300 mg/kg, i.p.) apparently suppresses tumor growth of HT-29 xenografts in athymic female mice by day 21. The two groups of Irinotecan (125 mg/kg) plus TSP-1 (10 mg/kg per day) or Irinotecan (150 mg/kg) in combination TSP-1 (20 mg/kg per day) are nearly equally effective and inhibit tumor growth 84% and 89%, respectively, and both are more effective than Irinotecan alone at doses of 250 and 300 mg/kg[3].
References:
[1]. Morales C, et al. Antitumoral effect of irinotecan (CPT-11) on an experimental model of malignant neuroectodermal tumor. J Neurooncol. 2002 Feb;56(3):219-26.
[2]. Pavillard V, et al. Determinants of the cytotoxicity of irinotecan in two human colorectal tumor cell lines. Cancer Chemother Pharmacol. 2002 Apr;49(4):329-35. Epub 2002 Jan 30.
[3]. Allegrini G, et al. Thrombospondin-1 plus irinotecan: a novel antiangiogenic-chemotherapeutic combination that inhibits the growth of advanced human colon tumor xenografts in mice. Cancer Chemother Pharmacol. 2004 Mar;53(3):261-6. Epub 2003 Dec 5.
- TZ9
Catalog No.:BCC5547
CAS No.:1002789-86-7
- Picrasidine J
Catalog No.:BCN5820
CAS No.:100234-62-6
- Picrasidine I
Catalog No.:BCN5819
CAS No.:100234-59-1
- AMG-208
Catalog No.:BCC1054
CAS No.:1002304-34-8
- Camstatin
Catalog No.:BCC5690
CAS No.:1002295-95-5
- 5,5'-Dimethoxysecoisolariciresinol
Catalog No.:BCN7941
CAS No.:1002106-91-3
- 2,3-dihydroxy-3-(4-hydroxyphenyl)propanoic acid
Catalog No.:BCN1641
CAS No.:100201-57-8
- Piscidinol A
Catalog No.:BCN5818
CAS No.:100198-09-2
- MK-8033
Catalog No.:BCC1768
CAS No.:1001917-37-8
- INH6
Catalog No.:BCC5455
CAS No.:1001753-24-7
- SRT1720 HCl
Catalog No.:BCC2222
CAS No.:1001645-58-4
- BV6
Catalog No.:BCC5372
CAS No.:1001600-56-1
- Apiopaeonoside
Catalog No.:BCN2801
CAS No.:100291-86-9
- Pemirolast potassium
Catalog No.:BCC4532
CAS No.:100299-08-9
- L(+)-Rhamnose monohydrate
Catalog No.:BCN8368
CAS No.:10030-85-0
- Curcumenone
Catalog No.:BCN3008
CAS No.:100347-96-4
- Calcium chloride dihydrate
Catalog No.:BCC7582
CAS No.:10035-04-8
- GSK 1562590 hydrochloride
Catalog No.:BCC8010
CAS No.:1003878-07-6
- Sodium Picosulfate
Catalog No.:BCC4845
CAS No.:10040-45-6
- Danshinspiroketallactone
Catalog No.:BCN3754
CAS No.:100414-80-0
- Lercanidipine
Catalog No.:BCC5239
CAS No.:100427-26-7
- Boric acid
Catalog No.:BCC7592
CAS No.:10043-35-3
- Cobicistat (GS-9350)
Catalog No.:BCC2271
CAS No.:1004316-88-4
- Dihydroresveratrol 3-O-glucoside
Catalog No.:BCN5821
CAS No.:100432-87-9
Inhibition of intestinal microflora beta-glucuronidase modifies the distribution of the active metabolite of the antitumor agent, irinotecan hydrochloride (CPT-11) in rats.[Pubmed:9744772]
Cancer Chemother Pharmacol. 1998;42(4):280-6.
PURPOSE: SN-38, a metabolite of Irinotecan hydrochloride (CPT-11), is considered to play a key role in the development of diarrhea as well as in the antitumor activity of CPT-11. We have previously found that the inhibition of beta-glucuronidase, which hydrolyzes detoxified SN-38 (SN-38 glucuronide) to reform SN-38, in the lumen by eliminating the intestinal microflora with antibiotics, markedly ameliorates the intestinal toxicity of CPT-11 in rats. In this study we compared the disposition of CPT-11 and its metabolites in rats treated with and without antibiotics. METHODS: Rats were given drinking water containing 1 mg/ml penicillin and 2 mg/ml streptomycin from 5 days before the administration of CPT-11 (60 mg/kg i.v.) and throughout the experiment. CPT-11, SN-38 glucuronide and SN-38 concentrations in the blood, intestinal tissues and intestinal luminal contents were determined by HPLC. RESULTS: Antibiotics had little or no effect on the pharmacokinetics of CPT-11, SN-38 glucuronide or SN-38 in the blood, or in the tissues or contents of the small intestine, which has less beta-glucuronidase activity in its luminal contents. In contrast, antibiotics markedly reduced the AUC1-24 h of SN-38 (by about 85%) in the large intestine tissue without changing that of CPT-11, and this was accompanied by a complete inhibition of the deconjugation of SN-38 glucuronide in the luminal contents. CONCLUSIONS: These results suggest that SN-38, which results from the hydrolysis of SN-38 glucuronide by beta-glucuronidase in the intestinal microflora, contributes considerably to the distribution of SN-38 in the large intestine tissue, and that inhibition of the beta-glucuronidase activity by antibiotics results in decreased accumulation of SN-38 in the large intestine.
Activity of CPT-11 (irinotecan hydrochloride), a topoisomerase I inhibitor, against human tumor colony-forming units.[Pubmed:8049503]
Anticancer Drugs. 1994 Apr;5(2):202-6.
CPT-11 (Irinotecan hydrochloride, 7-ethyl-10-[4-(piperidino)-1-piperidino] carbonyloxy-camptothecin) is a semisynthetic camptothecin derivative developed in Japan. The inhibitory activity of CPT-11 against human tumor colony-forming units from freshly explanted human tumors was explored using a soft agar cloning system. Final CPT-11 concentrations of 0.3-3.0 micrograms/ml were used for a 1 h exposure. At a concentration of 3.0 micrograms/ml antitumor activity was seen against colorectal, ovarian, nonsmall-cell lung, breast cancer and mesothelioma colony-forming units. CPT-11 should have activity against a broad spectrum of tumors in patients.
Therapeutic activity of CPT-11, a DNA-topoisomerase I inhibitor, against peripheral primitive neuroectodermal tumour and neuroblastoma xenografts.[Pubmed:8761367]
Br J Cancer. 1996 Aug;74(4):537-45.
The anti-tumour activity of CPT-11, a topoisomerase I inhibitor, was evaluated in four human neural-crest-derived paediatric tumour xenografts; one peripheral primitive neuroectodermal tumour (pPNET) (SK-N-MC) and three neuroblastomas. Two models, SK-N-MC and IGR-N835, were established in athymic mice from a previously established in vitro cell line. Two new neuroblastoma xenograft models, IGR-NB3 and IGR-NB8, were derived from previously untreated non-metastatic neuroblastomas. They exhibited the classic histological features of immature neuroblastoma along with N-myc amplification, paradiploidy, chromosome 1p deletions and overexpression of the human mdr 1 gene. These tumour markers have been shown to be poor prognostic factors in children treated for neuroblastoma. CPT-11 was tested against advanced stage subcutaneous tumours. CPT-11 was administered i.v. using an intermittent (q4d x 3) and a daily x 5 schedule. The optimal dosage and schedule was 40 mg kg-1 daily for 5 days. At this highest non-toxic dose, CPT-11 induced 100% tumour-free survivors on day 121 in mice bearing the pPNET SK-N-MC xenograft. For the three neuroblastoma xenografts, 38-100% complete tumour regressions were observed with a tumour growth delay from 38 to 42 days, and anti-tumour activity was clearly sustained at a lower dosage (27 mg kg-1 day-1). The efficacy of five anti-cancer drugs commonly used in paediatric oncology or in clinical development was evaluated against SK-N-MC and IGR-N835. The sensitivity of these two xenografts to cyclophosphamide, thiotepa and cisplatin was of the same order of magnitude as that of CPT-11, but they were refractory to etoposide and taxol. In conclusion, CPT-11 demonstrated significant activity against pPNET and neuroblastoma xenografts. Further clinical development of CPT-11 in paediatric oncology is warranted.
Inhibitory activity of camptothecin derivatives against acetylcholinesterase in dogs and their binding activity to acetylcholine receptors in rats.[Pubmed:8099964]
J Pharm Pharmacol. 1993 May;45(5):444-8.
A camptothecin derivative, 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin (CPT-11), shows a potent antitumour activity in experimental tumour models and in clinical trials. However, CPT-11 induced early diarrhoea and vomiting at high dose levels in clinical studies and showed an acetylcholine-like action on the guinea-pig ileum and trachea. In the present study, we investigated the activities of camptothecin derivatives in inhibiting acetylcholinesterase (AChE) and in binding to muscarinic acetylcholine receptors (AChR). CPT-11 inhibited AChE and binding of the specific ligand to AChR with respective 50% inhibition concentrations of 0.2 and 5 microM. These inhibitions were induced by camptothecin derivatives having an amino group at the C-10 position (or the C-4 position of hexacyclic derivatives), but were not or were only slightly induced by the others. Early defecation and vomiting in dogs were observed after intravenous injection of DU-6596 and DU-6888, two hexacyclic derivatives having the aminomethyl group at the C-4 position, and of CPT-11. DU-6174, however, which has a hydroxy group at this position, induced no early defecation and little vomiting. Plasma concentrations of CPT-11, DU-6596 and DU-6888 after intravenous treatment at doses causing such early adverse effects were maintained for 1 h or longer at levels sufficient to inhibit AChE. These results suggest that the inhibition of AChE by camptothecin derivatives with an amino group at the C-10 position (or the C-4 position) relates to the early defecation or diarrhoea and vomiting.


