Indole-3-acetic acidCAS# 87-51-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 87-51-4 | SDF | Download SDF |
| PubChem ID | 802 | Appearance | Powder |
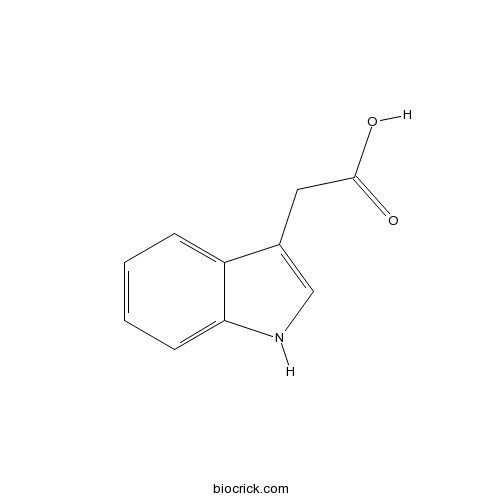
| Formula | C10H9NO2 | M.Wt | 175.18 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 30 mg/mL (171.25 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-(1~{H}-indol-3-yl)acetic acid | ||
| SMILES | C1=CC=C2C(=C1)C(=CN2)CC(=O)O | ||
| Standard InChIKey | SEOVTRFCIGRIMH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H9NO2/c12-10(13)5-7-6-11-9-4-2-1-3-8(7)9/h1-4,6,11H,5H2,(H,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Indole-3-acetic acid , a well-known phytohormone, can also be a signaling molecule in bacteria and therefore can have a direct effect on bacterial physiology. |
| In vitro | Indole-3-acetic acid in microbial and microorganism-plant signaling.[Pubmed: 17509086 ]FEMS Microbiol Rev. 2007 Jul;31(4):425-48.Diverse bacterial species possess the ability to produce the auxin phytohormone Indole-3-acetic acid (IAA).
Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid.[Pubmed: 15659623 ]Plant Cell. 2005 Feb;17(2):616-27. Epub 2005 Jan 19.Substantial evidence indicates that amino acid conjugates of Indole-3-acetic acid (IAA) function in auxin homeostasis, yet the plant enzymes involved in their biosynthesis have not been identified. Indole‐3‐acetic acid homeostasis in transgenic tobacco plants expressing the Agrobacterium rhizogenes rolB gene[Reference: WebLink]The Plant Journal, 2005, 3(5):681-689.The rolB gene of the plant pathogen Agrobacterium rhizogenes has an important role in the establishment of hairy root disease in infected plant tissues. When expressed as a single gene in transgenic plants the RolB protein gives rise to effects indicative of increased auxin activity. It has been reported that the RolB product is a β-glucosidase and proposed that the physiological and developmental alterations in transgenic plants expressing the rolB gene are the result of this enzyme hydrolysing bound auxins, in particular (indole-3-acetyl)-β-D-glucoside (IAGluc), and thereby bringing about an increase in the intracellular concentration of Indole-3-acetic acid (IAA).
|

Indole-3-acetic acid Dilution Calculator

Indole-3-acetic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.7084 mL | 28.5421 mL | 57.0841 mL | 114.1683 mL | 142.7104 mL |
| 5 mM | 1.1417 mL | 5.7084 mL | 11.4168 mL | 22.8337 mL | 28.5421 mL |
| 10 mM | 0.5708 mL | 2.8542 mL | 5.7084 mL | 11.4168 mL | 14.271 mL |
| 50 mM | 0.1142 mL | 0.5708 mL | 1.1417 mL | 2.2834 mL | 2.8542 mL |
| 100 mM | 0.0571 mL | 0.2854 mL | 0.5708 mL | 1.1417 mL | 1.4271 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-O-Acetyl-20-hydroxyecdysone
Catalog No.:BCN8715
CAS No.:19536-25-5
- Glycinol
Catalog No.:BCN8714
CAS No.:69393-95-9
- Bryonamide A
Catalog No.:BCN8713
CAS No.:75268-14-3
- Methyl caffeate acid
Catalog No.:BCN8712
CAS No.:3843-74-1
- Quercetin 3-O-beta-(6''-p-coumaroyl)glucopyranosyl(1->2)-alpha-L-rhamnopyranoside
Catalog No.:BCN8711
CAS No.:143061-65-8
- Asiaticoside B
Catalog No.:BCN8709
CAS No.:125265-68-1
- Isocarlinoside
Catalog No.:BCN8708
CAS No.:83151-90-0
- Pilosidine
Catalog No.:BCN8707
CAS No.:229971-57-7
- Trikamsteroside E
Catalog No.:BCN8706
CAS No.:952579-37-2
- 5,6,7,4'-tetrahydroxyisoflavone-6,7-di-O-beta-D-glucopyranoside
Catalog No.:BCN8705
CAS No.:1219001-04-3
- Xanthoangelol B
Catalog No.:BCN8704
CAS No.:132998-81-3
- Isololiolide
Catalog No.:BCN8703
CAS No.:38274-00-9
- 1-Isomangostin
Catalog No.:BCN8717
CAS No.:19275-44-6
- Nagarine
Catalog No.:BCN8718
CAS No.:41849-35-8
- 3-Methylxanthine
Catalog No.:BCN8719
CAS No.:1076-22-8
- 19 alpha-Hydroxyasiatic acid
Catalog No.:BCN8720
CAS No.:70868-78-9
- Dihydrowithaferin A
Catalog No.:BCN8721
CAS No.:5589-41-3
- Oleuroside
Catalog No.:BCN8722
CAS No.:116383-31-4
- Trikamsteroside C
Catalog No.:BCN8724
CAS No.:952579-35-0
- Pseudolaric acid C2
Catalog No.:BCN8726
CAS No.:82508-35-8
- 3-O-Acetyl-20-hydroxyecdysone
Catalog No.:BCN8728
CAS No.:22961-68-8
- N-Benzyloctadecanamide
Catalog No.:BCN8725
CAS No.:5327-45-7
- Hamaudol
Catalog No.:BCN6371
CAS No.:735-46-6
- Celosin I
Catalog No.:BCN8723
CAS No.:1807732-38-2


