Asiaticoside BCAS# 125265-68-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 125265-68-1 | SDF | Download SDF |
| PubChem ID | 91618002 | Appearance | White powder |
| Formula | C48H78O20 | M.Wt | 975.13 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Asiaticoside B | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
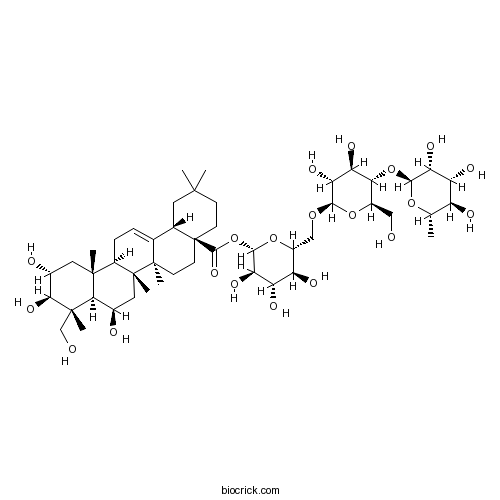
| Chemical Name | [(2~{S},3~{R},4~{S},5~{S},6~{R})-6-[[(2~{R},3~{R},4~{R},5~{S},6~{R})-3,4-dihydroxy-6-(hydroxymethyl)-5-[(2~{S},3~{R},4~{R},5~{R},6~{S})-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxymethyl]-3,4,5-trihydroxyoxan-2-yl] (4~{a}~{S},6~{a}~{R},6~{a}~{S},6~{b}~{R},8~{R},8~{a}~{R},9~{R},10~{R},11~{R},12~{a}~{R},14~{b}~{S})-8,10,11-trihydroxy-9-(hydroxymethyl)-2,2,6~{a},6~{b},9,12~{a}-hexamethyl-1,3,4,5,6,6~{a},7,8,8~{a},10,11,12,13,14~{b}-tetradecahydropicene-4~{a}-carboxylate | ||
| SMILES | CC1C(C(C(C(O1)OC2C(OC(C(C2O)O)OCC3C(C(C(C(O3)OC(=O)C45CCC(CC4C6=CCC7C8(CC(C(C(C8C(CC7(C6(CC5)C)C)O)(C)CO)O)O)C)(C)C)O)O)O)CO)O)O)O | ||
| Standard InChIKey | NNWMHSNRRWMMBI-PJISEHJASA-N | ||
| Standard InChI | InChI=1S/C48H78O20/c1-20-28(53)30(55)33(58)40(64-20)67-36-25(17-49)65-39(35(60)32(36)57)63-18-26-29(54)31(56)34(59)41(66-26)68-42(62)48-12-10-43(2,3)14-22(48)21-8-9-27-44(4)15-24(52)38(61)45(5,19-50)37(44)23(51)16-47(27,7)46(21,6)11-13-48/h8,20,22-41,49-61H,9-19H2,1-7H3/t20-,22-,23+,24+,25+,26+,27+,28-,29+,30+,31-,32+,33+,34+,35+,36+,37+,38-,39+,40-,41-,44+,45-,46+,47+,48-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Asiaticoside B has notable cytotoxicity against HepG2 and MCF-7 cancer cell lines. |
| In vitro | Cytotoxic cycloartane triterpene saponins from Actaea asiatica.[Pubmed: 17067171 ]J Nat Prod. 2006 Oct;69(10):1500-2.
|
| Structure Identification | Pharmazie. 2003 Jun;58(6):381-4.An improved HPLC method for quantitative determination of six triterpenes in Centella asiatica extracts and commercial products.[Pubmed: 12856998]
|

Asiaticoside B Dilution Calculator

Asiaticoside B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0255 mL | 5.1275 mL | 10.255 mL | 20.5101 mL | 25.6376 mL |
| 5 mM | 0.2051 mL | 1.0255 mL | 2.051 mL | 4.102 mL | 5.1275 mL |
| 10 mM | 0.1026 mL | 0.5128 mL | 1.0255 mL | 2.051 mL | 2.5638 mL |
| 50 mM | 0.0205 mL | 0.1026 mL | 0.2051 mL | 0.4102 mL | 0.5128 mL |
| 100 mM | 0.0103 mL | 0.0513 mL | 0.1026 mL | 0.2051 mL | 0.2564 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isocarlinoside
Catalog No.:BCN8708
CAS No.:83151-90-0
- Pilosidine
Catalog No.:BCN8707
CAS No.:229971-57-7
- Trikamsteroside E
Catalog No.:BCN8706
CAS No.:952579-37-2
- 5,6,7,4'-tetrahydroxyisoflavone-6,7-di-O-beta-D-glucopyranoside
Catalog No.:BCN8705
CAS No.:1219001-04-3
- Xanthoangelol B
Catalog No.:BCN8704
CAS No.:132998-81-3
- Isololiolide
Catalog No.:BCN8703
CAS No.:38274-00-9
- Bryonamide B
Catalog No.:BCN8702
CAS No.:5942-25-6
- Cinchonine hydrochloride
Catalog No.:BCN8701
CAS No.:5949-11-1
- Tangshenoside I
Catalog No.:BCN8700
CAS No.:117278-74-7
- Trillikamtoside R
Catalog No.:BCN8699
CAS No.:2098642-71-6
- Secologanoside
Catalog No.:BCN8698
CAS No.:59472-23-0
- Picropodophyllol
Catalog No.:BCN8697
CAS No.:3811-15-2
- Quercetin 3-O-beta-(6''-p-coumaroyl)glucopyranosyl(1->2)-alpha-L-rhamnopyranoside
Catalog No.:BCN8711
CAS No.:143061-65-8
- Methyl caffeate acid
Catalog No.:BCN8712
CAS No.:3843-74-1
- Bryonamide A
Catalog No.:BCN8713
CAS No.:75268-14-3
- Glycinol
Catalog No.:BCN8714
CAS No.:69393-95-9
- 2-O-Acetyl-20-hydroxyecdysone
Catalog No.:BCN8715
CAS No.:19536-25-5
- Indole-3-acetic acid
Catalog No.:BCN8716
CAS No.:87-51-4
- 1-Isomangostin
Catalog No.:BCN8717
CAS No.:19275-44-6
- Nagarine
Catalog No.:BCN8718
CAS No.:41849-35-8
- 3-Methylxanthine
Catalog No.:BCN8719
CAS No.:1076-22-8
- 19 alpha-Hydroxyasiatic acid
Catalog No.:BCN8720
CAS No.:70868-78-9
- Dihydrowithaferin A
Catalog No.:BCN8721
CAS No.:5589-41-3
- Oleuroside
Catalog No.:BCN8722
CAS No.:116383-31-4
New 9, 19-cycloartane triterpenoid from the root of Cimicifuga foetida.[Pubmed:24863355]
Chin J Nat Med. 2014 Apr;12(4):294-6.
AIM: To study the 9, 19-cycloartane triterpenes from the roots of Cimicifuga foetida. METHOD: Chromatographic separations by silica gel, C18 reversed phase silica gel, and high-performance liquid chromatography (HPLC) were used. All of the structures were elucidated on the basis of spectroscopic analysis and chemical methods. RESULTS: Five 9, 19-cycloartane triterpenes, (3beta, 12beta, 15alpha, 24R)-12, 2'-diacetoxy-24, 25-epoxy-15-hydroxy-16, 23-dione-3-O-alpha-L-arabinopyranoside (1), actein (2), 23-epi-26-deoxyactein (3), Asiaticoside B (4), and 12beta-hydroxycimigenol (5) were isolated from the roots of Cimicifuga foetida. CONCLUSION: Compound 1 is a new triterpene with two acetoxy groups at C-2' and C-12.
Cytotoxic cycloartane triterpene saponins from Actaea asiatica.[Pubmed:17067171]
J Nat Prod. 2006 Oct;69(10):1500-2.
Three new 9,19-cycloartane triterpene glycosides, asiaticoside A (1), Asiaticoside B (2), and 25-O-ethylcimigenol-3-O-beta-D-xylopyranoside (3), together with cimiacemoside I (4), 25-O-acetylcimigenol-3-beta-O-D-xyloside (5), and 25-anhydrocimigenol-beta-O-D-xyloside (6) were isolated from the roots/rhizomes extract of Actaea asiatica, and their structures were established by spectroscopic methods (IR, HRESIMS, and NMR). Compounds 1-3, 5, and 6 had notable cytotoxicity against HepG2 and MCF-7 cancer cell lines.
Medicinal foodstuffs. XXVII. Saponin constituents of gotu kola (2): structures of new ursane- and oleanane-type triterpene oligoglycosides, centellasaponins B, C, and D, from Centella asiatica cultivated in Sri Lanka.[Pubmed:11605675]
Chem Pharm Bull (Tokyo). 2001 Oct;49(10):1368-71.
Ursane- and oleanane-type triterpene oligoglycosides, centellasaponins B, C, and D, were isolated from the aerial parts of Centella asiatica (L.) Urban cultivated in Sri Lanka together with madecassoside, asiaticoside, Asiaticoside B, and sceffoleoside A. The chemical structures of centellasaponins B, C, and D were determined on the basis of chemical and physicochemical evidence to be madecassic acid 28-O-beta-D-glucopyranosyl(1-->6)-beta-D-glucopyranoside, madasiatic acid 28-O-alpha-L-rhamnopyranosyl(1-->4)-beta-D-glucopyranosyl(1-->6)-beta-D-glucopyra noside, and 3beta,6beta,23-trihydroxyolean-12-en-28-oic acid 28-O-alpha-L-rhamnopyranosyl(1-->4)-beta-D-glucopyranosyl(1-->6)-beta-D-glucopyra noside, respectively.


