HotrienolCAS# 20053-88-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20053-88-7 | SDF | Download SDF |
| PubChem ID | 5366264 | Appearance | Oil |
| Formula | C10H16O | M.Wt | 152.23 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
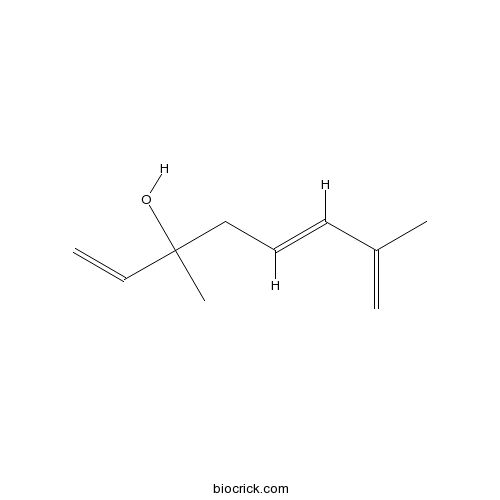
| Synonyms | 3,7-Dimethyl-1,5,7-octatrien-3-ol | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (5E)-3,7-dimethylocta-1,5,7-trien-3-ol | ||
| SMILES | CC(=C)C=CCC(C)(C=C)O | ||
| Standard InChIKey | ZJIQIJIQBTVTDY-VOTSOKGWSA-N | ||
| Standard InChI | InChI=1S/C10H16O/c1-5-10(4,11)8-6-7-9(2)3/h5-7,11H,1-2,8H2,3-4H3/b7-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Hotrienol has antioxidant capacity. 2. Hotrienol is an excellent fruity smelling compound,could be a flavouring ingredient. 3. Hotrienol and methyl syringate can be considered non-specific chemical markers of S. hortensis honey. |

Hotrienol Dilution Calculator

Hotrienol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.569 mL | 32.845 mL | 65.6901 mL | 131.3801 mL | 164.2252 mL |
| 5 mM | 1.3138 mL | 6.569 mL | 13.138 mL | 26.276 mL | 32.845 mL |
| 10 mM | 0.6569 mL | 3.2845 mL | 6.569 mL | 13.138 mL | 16.4225 mL |
| 50 mM | 0.1314 mL | 0.6569 mL | 1.3138 mL | 2.6276 mL | 3.2845 mL |
| 100 mM | 0.0657 mL | 0.3285 mL | 0.6569 mL | 1.3138 mL | 1.6423 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GMX1778 (CHS828)
Catalog No.:BCC6527
CAS No.:200484-11-3
- Picralinal
Catalog No.:BCN4878
CAS No.:20045-06-1
- UB 165 fumarate
Catalog No.:BCC5746
CAS No.:200432-86-6
- 6-Chloroguanine riboside
Catalog No.:BCC8771
CAS No.:2004-07-1
- Fmoc-D-Cys(Trt)-OPfp
Catalog No.:BCC3482
CAS No.:200395-72-8
- Fmoc-D-Cit-OH
Catalog No.:BCC3181
CAS No.:200344-33-8
- Fmoc-D-Asp(OtBu)-Opfp
Catalog No.:BCC3472
CAS No.:200335-75-7
- Boc-D-Asp(OtBu)-OH.DCHA
Catalog No.:BCC3373
CAS No.:200334-95-8
- Melanocyte stimulating hormone release inhibiting factor
Catalog No.:BCC1043
CAS No.:2002-44-0
- Z-Arg(Pbf)-OH.CHA
Catalog No.:BCC3064
CAS No.:200190-89-2
- CGP 3466B maleate
Catalog No.:BCC5955
CAS No.:200189-97-5
- Fmoc-Arg(Pbf)-OPfp
Catalog No.:BCC3041
CAS No.:200132-16-7
- Fmoc-D-Glu(OtBu)-OPfp
Catalog No.:BCC3497
CAS No.:200616-21-3
- Fmoc-N-Me-Glu(OtBu)-OH
Catalog No.:BCC3213
CAS No.:200616-40-6
- Fmoc-D-Gln-OPfp
Catalog No.:BCC3487
CAS No.:200622-33-9
- Fmoc-D-Gln(Trt)-OH
Catalog No.:BCC3488
CAS No.:200623-62-7
- Hennadiol
Catalog No.:BCN4679
CAS No.:20065-99-0
- Piplartine
Catalog No.:BCN4037
CAS No.:20069-09-4
- (-)-Phyllocladene
Catalog No.:BCN7661
CAS No.:20070-61-5
- 7-Hydroxy-PIPAT maleate
Catalog No.:BCC6760
CAS No.:200722-46-9
- 16-Nor-15-oxodehydroabietic acid
Catalog No.:BCN3943
CAS No.:200813-31-6
- Pseudoneolinderane
Catalog No.:BCN8034
CAS No.:20082-45-5
- Epicurzerenone
Catalog No.:BCN3521
CAS No.:20085-85-2
- Diosbulbin B
Catalog No.:BCN4879
CAS No.:20086-06-0
Monitoring the evolution of volatile compounds using gas chromatography during the stages of production of Moscatel sparkling wine.[Pubmed:25863638]
Food Chem. 2015 Sep 15;183:291-304.
This study reports, for the first time, the main changes that occur with some important aroma compounds of Moscatel sparkling wines during winemaking, measured using headspace solid-phase microextraction, one-dimensional and comprehensive two-dimensional gas chromatography (GCxGC) with mass spectrometry detection (MS). The best conditions of volatile extraction included the use of PDMS/DVB fibre, 2mL of wine, 30% of NaCl, 40 degrees C for 30min. The chromatographic profile of sparkling wines showed decreasing amounts of monoterpenes (limonene, 4-terpineol, terpinolene, citronellol, alpha-terpineol, linalool, Hotrienol, and nerol oxide), increasing amounts of esters (terpenyl esters, ethyl octanoate, ethyl decanoate and hexyl acetate) and alcohols (1-nonanol and 2-phenylethanol). Sixty-nine compounds co-eluted in the first dimension; only six co-eluted in the second dimension. GCxGC/TOFMS allows more detailed study of the volatile profile of sparkling wines.
Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea.[Pubmed:25148991]
Food Chem. 2015 Jan 15;167:290-8.
The aroma constituents of Kangra orthodox black tea were isolated by simultaneous distillation extraction (SDE), supercritical fluid extraction and beverage method. The aroma-active compounds were identified using gas chromatography-olfactometry-mass spectrometry. Geraniol, linalool, (Z/E)-linalool oxides, (E)-2-hexenal, phytol, beta-ionone, Hotrienol, methylpyrazine and methyl salicylate were major volatile constituents in all the extracts. Minor volatile compounds in all the extracts were 2-ethyl-5-methylpyrazine, ethylpyrazine, 2-6,10,14-trimethyl-2-pentadecanone, acetylfuran, hexanoic acid, dihydroactinidiolide and (E/Z)-2,6-nonadienal. The concentrated SDE extract was fractionated into acidic, basic, water-soluble and neutral fractions. The neutral fraction was further chromatographed on a packed silica gel column eluted with pentane and diethyl ether to separate minor compounds. The aroma-active compounds identified using gas chromatography-olfactometry-mass spectrometry were 2-amylfuran, (E/Z)-2,6-nonadienal, 1-pentanol, epoxylinalool, (Z)-jasmone, 2-acetylpyrrole, farnesyl acetone, geranyl acetone, cadinol, cubenol and dihydroactinidiolide. AEDA studies showed 2-hexenal, 3-hexenol, ethylpyrazine, (Z/E)-linalool oxides, linalool, (E/Z)-2,6-nonadienal, geraniol, phenylethanol, beta-ionone, Hotrienol and dihydroactinidiolide to be odour active components.
Antioxidant capacity and chemical profiles of Satureja montana L. Honey: hotrienol and syringyl derivatives as biomarkers.[Pubmed:26172325]
Chem Biodivers. 2015 Jul;12(7):1047-56.
The present study is focused on the antioxidant capacity and chemical profiling of eight Croatian Satureja montana L. honey samples. Among the 20 compounds obtained by headspace solid-phase microextraction (HS-SPME) and identified by GC-FID and GC/MS analyses, Hotrienol was predominant (75.9-81.7%). The honey matrix volatile/semivolatile profile was investigated by ultrasonic solvent extraction (USE) followed by GC-FID and GC/MS analyses. The major compounds identified by this latter method were the sinapic-acid derivatives methyl syringate (36.2-72.8%) and syringaldehyde (2.2-43.1%). Direct, targeted HPLC-DAD analyses of the native honey samples revealed the presence of methyl syringate (7.10-39.60 mg/kg) and syringic acid (0.10-1.70 mg/kg). In addition, the total phenolic content of the samples was determined by the FolinCiocalteu assay (311.0-465.9 mg GAE/kg), and the antioxidant capacity was evaluated by the DPPH radical-scavenging activity (0.5-1.0 mmol TEAC/kg) and the ferric reducing antioxidant power (2.5-5.1 mmol Fe(2+) /kg).
A practical and convenient synthesis of hotrienol, an excellent fruity smelling compound.[Pubmed:12822943]
J Agric Food Chem. 2003 Jul 2;51(14):4036-9.
The practical and convenient synthesis of Hotrienol, which is an excellent fruity smelling compound, has been performed by the ene-type chlorination of linalyl acetate and then dehydrochlorinated by lithium bromide and lithium carbonate in DMF, followed by hydrolysis in three steps with an overall yield of 55%.


