HomopiperazineCAS# 505-66-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 505-66-8 | SDF | Download SDF |
| PubChem ID | 68163 | Appearance | Powder |
| Formula | C5H12N2 | M.Wt | 100.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
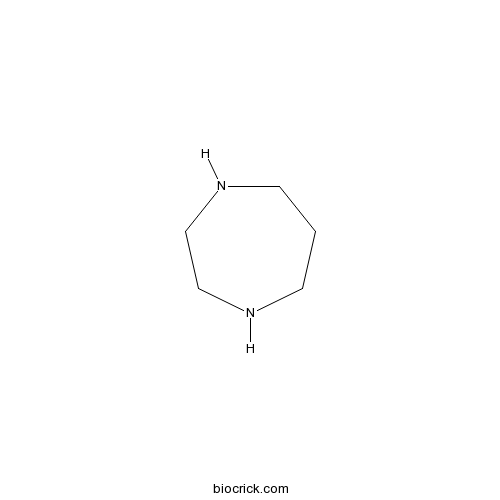
| Chemical Name | 1,4-diazepane | ||
| SMILES | C1CNCCNC1 | ||
| Standard InChIKey | FQUYSHZXSKYCSY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H12N2/c1-2-6-4-5-7-3-1/h6-7H,1-5H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Homopiperazine Dilution Calculator

Homopiperazine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 9.98 mL | 49.9002 mL | 99.8004 mL | 199.6008 mL | 249.501 mL |
| 5 mM | 1.996 mL | 9.98 mL | 19.9601 mL | 39.9202 mL | 49.9002 mL |
| 10 mM | 0.998 mL | 4.99 mL | 9.98 mL | 19.9601 mL | 24.9501 mL |
| 50 mM | 0.1996 mL | 0.998 mL | 1.996 mL | 3.992 mL | 4.99 mL |
| 100 mM | 0.0998 mL | 0.499 mL | 0.998 mL | 1.996 mL | 2.495 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Araneosol
Catalog No.:BCN5613
CAS No.:50461-86-4
- GW441756
Catalog No.:BCC5093
CAS No.:504433-23-2
- Methyl 2alpha-hydroxyhardwickiate
Catalog No.:BCN7595
CAS No.:50428-93-8
- 1,5,6-Trihydroxyxanthone
Catalog No.:BCN7642
CAS No.:5042-03-5
- Isorhamnetin-3-O-beta-D-Glucoside
Catalog No.:BCN1247
CAS No.:5041-82-7
- Isoliquiritin
Catalog No.:BCN5945
CAS No.:5041-81-6
- Juglanin
Catalog No.:BCN6505
CAS No.:5041-67-8
- 3-Nitropropionic acid
Catalog No.:BCC6303
CAS No.:504-88-1
- DL-Homocysteic acid
Catalog No.:BCN2233
CAS No.:504-33-6
- 4-Aminopyridine
Catalog No.:BCC5267
CAS No.:504-24-5
- Orcinol
Catalog No.:BCN5612
CAS No.:504-15-4
- (-)-di-de-Omethylgrandisin
Catalog No.:BCN7872
CAS No.:50393-98-1
- 3-(Carboxymethylamino)propanoic acid
Catalog No.:BCN1791
CAS No.:505-72-6
- Fenspiride HCl
Catalog No.:BCC4659
CAS No.:5053-08-7
- Columbianadin
Catalog No.:BCN1275
CAS No.:5058-13-9
- Isojacareubin
Catalog No.:BCN6883
CAS No.:50597-93-8
- Arachidonic acid
Catalog No.:BCN2215
CAS No.:506-32-1
- Nervonic acid
Catalog No.:BCN8374
CAS No.:506-37-6
- Octacosanoic Acid
Catalog No.:BCN5395
CAS No.:506-48-9
- Niranthin
Catalog No.:BCN5614
CAS No.:50656-77-4
- Alkaloid KD1
Catalog No.:BCN1898
CAS No.:50656-87-6
- Alkaloid C
Catalog No.:BCN1897
CAS No.:50656-88-7
- Vandrikidine
Catalog No.:BCN5615
CAS No.:50656-92-3
- Chasmanine
Catalog No.:BCN5409
CAS No.:5066-78-4
Homopiperazine-rhodamine B adducts of triterpenoic acids are strong mitocans.[Pubmed:29960206]
Eur J Med Chem. 2018 Jul 15;155:869-879.
Parent pentacyclic triterpenoic acids such as ursolic-, oleanolic, glycyrrhetinic, betulinic and boswellic acid were converted into their acetylated piperazinyl amides that were coupled with rhodamine B. SRB assays to evaluate their cytotoxicity showed all of these triterpene-homopiperazinyl-rhodamine adducts 16-20 being highly cytotoxic for a panel of human tumor cell lines even in nanomolar concentrations while being significantly less cytotoxic for non-malignant cells. Interestingly enough, these compounds were even more cytotoxic than previously prepared piperazinyl analogs, thus making the homopiperazinyl spacer a very interesting scaffold for the development of biologically active compounds. Extra staining experiments showed that the cytostatic effect of compounds 18 and 20 onto A2780 cancer cells is due to their ability to act as a mitocan.
Homopiperazine Derived Female Controlled Vaginal Trichomonacidal Contraceptive: An Addition to Structure-Activity Relationship.[Pubmed:29792148]
Med Chem. 2018;14(8):773-783.
BACKGROUND: In our previous work, several piperazine derived bis(dialkylaminethiocarbonyl) disulfides and disulfide esters of dithiocarbamic acid have been synthesized and evaluated for their spermicidal and microbicidal efficacy. These studies have provided some promising compounds for developing a dually active vaginal microbicidal contraceptive which is under pre-clinical stage. OBJECTIVE: The main objective of this study was the design synthesis and biological evaluation of bis(dialkylaminethiocarbonyl) disulfides (4-15) and 2,2'-disulfanediylbis (3-(substituted-1-yl) propane-2,1-diyl) disubstituted-1-carbodithioates (19-28) as non-surfactant molecules capable of eliminating Trichomonas vaginalis as well as irreversibly immobilizing 100% human sperm promptly. METHOD: Spermicidal, anti-trichomonas, cytotoxicity and biocompatibility study of the synthesized compounds was done as per the reported methodologies. RESULT: Among bis(dialkylaminethiocarbonyl) disulfides (4-15, Table 1), compound 4 (MEC 0.02 mM) was found to be the most desirable for spermicidal activity as it was 40 times more active than Nonoxynol-9 (N-9), and also active against Trichomonas vaginalis (MIC 0.02 &1.10 mM). 2, 2'-disulfanediylbis (3-(substituted- 1-yl) propane-2, 1-diyl) disubstituted-1-carbodithioates (19-28, Table 2), and compounds (19, 22, 23, and 24 MEC 0.05 mM) were sixteen times more active than N-9 with promising Trichomonacidal activity. CONCLUSION: This study suggested that the disulfide linkage alone and dithiocarbamate along with disulfide group within the same chemical entity impart the desired multiple activities of compounds.
Evaluation of buffers toxicity in tobacco cells: Homopiperazine-1,4-bis (2-ethanesulfonic acid) is a suitable buffer for plant cells studies at low pH.[Pubmed:28364708]
Plant Physiol Biochem. 2017 Jun;115:119-125.
Low pH is an important environmental stressor of plant root cells. Understanding the mechanisms of stress and tolerance to acidity is critical; however, there is no widely accepted pH buffer for studies of plant cells at low pH. Such a buffer might also benefit studies of Al toxicity, in which buffering at low pH is also important. The challenge is to find a buffer with minimal cellular effects. We examined the cytotoxicity and possible metabolic disturbances of four buffers that have adequate pKa values and potential use for studies in the pH range of 4.0-5.0. These were homopipes (Homopiperazine-1,4-bis (2-ethanesulfonic acid); pKa1 4.4), 3,3-dimethylglutaric acid (pKa1 3.73), beta-alanine (pKa1 3.70) and potassium biphthalate (pKa1 2.95; pKa2 5.41). First, tobacco BY-2 cells were grown in a rich medium containing 10 mM of each buffer or MES (2-(N-morpholino) ethanesulfonic acid) as a control, with the pH initially adjusted to 5.7. beta-alanine was clearly toxic and dimethylgluturate and biphthalate were found to be cytostatic, in which no culture growth occurred but cell viability was either unaffected or decreased only after 5 days. Only homopipes allowed normal culture growth and cell viability. Homopipes (10 mM) was then tested in cell cultures with an initial pH of 4.3 +/- 0.17 in minimal medium to examine whether its undissociated species (H2A) displayed any cellular effects and no cytotoxic effects were observed. It is possible to conclude that among tested buffers, homopipes is the most suitable for studies at low pH, and may be especially useful for aluminum toxicity experiments.


