GW441756TrkA inhibitor,potent and selective CAS# 504433-23-2 |
- Obeticholic Acid
Catalog No.:BCC5572
CAS No.:459789-99-2
- Chenodeoxycholic acid
Catalog No.:BCN2620
CAS No.:474-25-9
- XL335
Catalog No.:BCC4501
CAS No.:629664-81-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 504433-23-2 | SDF | Download SDF |
| PubChem ID | 16219401 | Appearance | Powder |
| Formula | C17H13N3O | M.Wt | 275.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 17.5 mg/mL (63.57 mM; Need ultrasonic and warming) H2O : < 0.1 mg/mL (insoluble) | ||
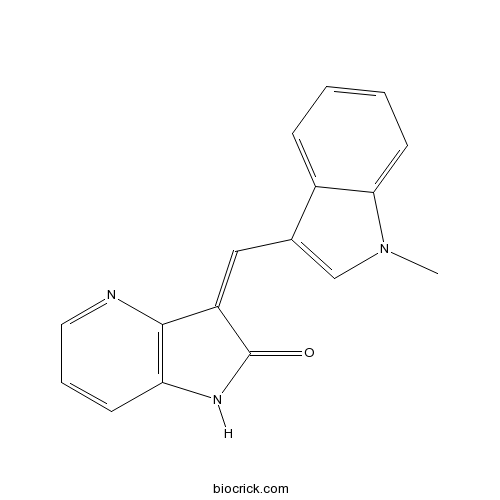
| Chemical Name | 3-[(1-methylindol-3-yl)methylidene]-1H-pyrrolo[3,2-b]pyridin-2-one | ||
| SMILES | CN1C=C(C2=CC=CC=C21)C=C3C4=C(C=CC=N4)NC3=O | ||
| Standard InChIKey | NXNQLECPAXXYTR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H13N3O/c1-20-10-11(12-5-2-3-7-15(12)20)9-13-16-14(19-17(13)21)6-4-8-18-16/h2-10H,1H3,(H,19,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, selective inhibitor of the NGF receptor tyrosine kinase A (TrkA) (IC50 = 2 nM). Displays > 100-fold selectivity over a range of other kinases. |

GW441756 Dilution Calculator

GW441756 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6324 mL | 18.162 mL | 36.324 mL | 72.648 mL | 90.81 mL |
| 5 mM | 0.7265 mL | 3.6324 mL | 7.2648 mL | 14.5296 mL | 18.162 mL |
| 10 mM | 0.3632 mL | 1.8162 mL | 3.6324 mL | 7.2648 mL | 9.081 mL |
| 50 mM | 0.0726 mL | 0.3632 mL | 0.7265 mL | 1.453 mL | 1.8162 mL |
| 100 mM | 0.0363 mL | 0.1816 mL | 0.3632 mL | 0.7265 mL | 0.9081 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GW441756 is an inhibitor of TrkA kinase and LRRK2 with IC50 value of 320nM and 2nM, respectively [1].
The activity of TrkA kinase can affect its downstream signaling and involves in many biological processes including proliferation, differentiation and apoptosis. Thereby the inhibitor of TrkA is developed for the treatment of cancers. GW441756 belongs to the oxindole series and is found to be a potent inhibitor of TrkA. It is also selective against TrkA. The IC50 values of it for cRaf1 and CDK2 are above 12μM and 7μM, respectively. In human muscle sarcoma cancer cell line HTB114, treatment of GW441756 dose-dependently suppresses neoplastic proliferation and induces apoptosis [1, 2].
In addition, GW441756 is also found to be an LRRK2 inhibitor. It inhibits the Ser935 phosphorylation of LRRK2 in cellular TR-FRET assay with IC50 value of 2.2μM. It shows no significant cytotoxicity with IC50 value of >20μM [3].
References:
[1] Wood E R, Kuyper L, Petrov K G, et al. Discovery and in vitro evaluation of potent TrkA kinase inhibitors: oxindole and aza-oxindoles. Bioorganic & medicinal chemistry letters, 2004, 14(4): 953-957.
[2] Montagnoli C, Pistilli A, Stabile A M, et al. Anti-proliferative effects of GW441756, a novel inhibitor of NGFreceptor tyrosine kinase a (TRKA), in human sarcoma. Italian Journal of Anatomy and Embryology, 2010, 115(1/2): 117.
[3] Hermanson S B, Carlson C B, Riddle S M, et al. Screening for novel LRRK2 inhibitors using a high-throughput TR-FRET cellular assay for LRRK2 Ser935 phosphorylation. PloS one, 2012, 7(8): e43580.
- Methyl 2alpha-hydroxyhardwickiate
Catalog No.:BCN7595
CAS No.:50428-93-8
- 1,5,6-Trihydroxyxanthone
Catalog No.:BCN7642
CAS No.:5042-03-5
- Isorhamnetin-3-O-beta-D-Glucoside
Catalog No.:BCN1247
CAS No.:5041-82-7
- Isoliquiritin
Catalog No.:BCN5945
CAS No.:5041-81-6
- Juglanin
Catalog No.:BCN6505
CAS No.:5041-67-8
- 3-Nitropropionic acid
Catalog No.:BCC6303
CAS No.:504-88-1
- DL-Homocysteic acid
Catalog No.:BCN2233
CAS No.:504-33-6
- 4-Aminopyridine
Catalog No.:BCC5267
CAS No.:504-24-5
- Orcinol
Catalog No.:BCN5612
CAS No.:504-15-4
- (-)-di-de-Omethylgrandisin
Catalog No.:BCN7872
CAS No.:50393-98-1
- Cyclo(Phe-Gly)
Catalog No.:BCN2431
CAS No.:5037-75-2
- Apixaban
Catalog No.:BCC2295
CAS No.:503612-47-3
- Araneosol
Catalog No.:BCN5613
CAS No.:50461-86-4
- Homopiperazine
Catalog No.:BCC8995
CAS No.:505-66-8
- 3-(Carboxymethylamino)propanoic acid
Catalog No.:BCN1791
CAS No.:505-72-6
- Fenspiride HCl
Catalog No.:BCC4659
CAS No.:5053-08-7
- Columbianadin
Catalog No.:BCN1275
CAS No.:5058-13-9
- Isojacareubin
Catalog No.:BCN6883
CAS No.:50597-93-8
- Arachidonic acid
Catalog No.:BCN2215
CAS No.:506-32-1
- Nervonic acid
Catalog No.:BCN8374
CAS No.:506-37-6
- Octacosanoic Acid
Catalog No.:BCN5395
CAS No.:506-48-9
- Niranthin
Catalog No.:BCN5614
CAS No.:50656-77-4
- Alkaloid KD1
Catalog No.:BCN1898
CAS No.:50656-87-6
- Alkaloid C
Catalog No.:BCN1897
CAS No.:50656-88-7
Distinct TrkA and Ret modulated negative and positive neuropathic behaviors in a mouse model of resiniferatoxin-induced small fiber neuropathy.[Pubmed:29106982]
Exp Neurol. 2018 Feb;300:87-99.
Neurotrophic factors and their corresponding receptors play key roles in the maintenance of different phenotypic dorsal root ganglion (DRG) neurons, the axons of which degenerate in small fiber neuropathy, leading to various neuropathic manifestations. Mechanisms underlying positive and negative symptoms of small fiber neuropathy have not been systematically explored. This study investigated the molecular basis of these seemingly paradoxical neuropathic behaviors according to the profiles of TrkA and Ret with immunohistochemical and pharmacological interventions in a mouse model of resiniferatoxin (RTX)-induced small fiber neuropathy. Mice with RTX neuropathy exhibited thermal hypoalgesia and mechanical allodynia, reduced skin innervation, and altered DRG expression profiles with decreased TrkA(+) neurons and increased Ret(+) neurons. RTX neuropathy induced the expression of activating transcription factor 3 (ATF3), and ATF3(+) neurons were colocalized with Ret but not with TrkA (P<0.001). As a neuroprotectant, 4-Methylcatechol (4MC) promoted skin reinnervation partially with correlated reversal of the neuropathic behaviors and altered neurochemical expression. Gambogic amide, a selective TrkA agonist, normalized thermal hypoalgesia, and GW441756, a TrkA kinase inhibitor, induced thermal hypoalgesia, which was already reversed by 4MC. Mechanical allodynia was reversed by a Ret kinase inhibitor, AST487, which induced thermal hyperalgesia in naive mice. The activation of Ret signaling by XIB4035 induced mechanical allodynia and thermal hypoalgesia in RTX neuropathy mice in which the neuropathic behaviors were previously normalized by 4MC. Distinct neurotrophic factor receptors, TrkA and Ret, accounted for negative and positive neuropathic behaviors in RTX-induced small fiber neuropathy, respectively: TrkA for thermal hypoalgesia and Ret for mechanical allodynia and thermal hypoalgesia.
Mechanism for neurotropic action of vorinostat, a pan histone deacetylase inhibitor.[Pubmed:27678157]
Mol Cell Neurosci. 2016 Dec;77:11-20.
In this study we investigated the neurotrophic actions of vorinostat (suberoylanilide hydroxamic acid, SAHA), a class I and class II HDAC inhibitor, on the differentiation of Neuroscreen-1 (NS-1) cells. NS-1 cell is a subclone of the rat pheochromocytoma cell line (PC 12). Vorinostat independently induced neurite outgrowth in NS-1 cells. The NS-1 cells were further interrogated for the effects of vorinostat on intracellular neurotrophin signaling pathways, to understand its mechanism of neurotrophic action. Selective inhibitors of MEK1/2 (PD98059 and U0126), phosphoinositide 3-kinase (PI3K) (LY294002) and tyrosine kinase A (TrkA) (GW441756) were employed for these interrogations. Our results suggest that neurite outgrowth mediated by both nerve growth factor (NGF), an intrinsic neurotrophin, and vorinostat were blocked by the inhibitors of MEK1/2 & PI3K. Vorinostat induced phosphorylation of ERK1/2 occurs at 2h post treatment. Phosphorylation of ERK was abolished in presence of U0126, further confirming the role of ERK pathway in vorinostat-induced differentiation of NS-1 cells. Vorinostat-induced neurite outgrowth also involves the activation of upstream extracellular kinase TrkA, as both vorinostat mediated neurite outgrowth and activation of ERK were attenuated in presence of the TrkA inhibitor, GW441756. Vorinostat also stimulated hyperacetylation of alpha-tubulin and histones H3/H4 in NS-1 cells. The results suggest that vorinostat exerts a positive effect on the neuritogenesis via activation of MEK1/2 & PI3K pathways involving an upstream kinase, TrkA. Bioactive small molecules with neurotrophic and neuritogenic actions, like vorinostat identified in the present study, hold great promise as therapeutic agents for treatment of neurodegenerative diseases and neuronal injuries by virtue of their ability to stimulate neuritic outgrowth.
Blocking Nerve Growth Factor Signaling Reduces the Neural Invasion Potential of Pancreatic Cancer Cells.[Pubmed:27792755]
PLoS One. 2016 Oct 28;11(10):e0165586.
Perineural invasion (PNI) is thought to be one of the factors responsible for the high rate of tumor recurrence after surgery and the pain generation associated with pancreatic cancer. Signaling via the nerve growth factor (NGF) pathway between pancreatic cancer cells and the surrounding nerves has been implicated in PNI, and increased levels of these proteins have been correlated to poor prognosis. In this study, we examine the molecular mechanism of the NGF signaling pathway in PNI in pancreatic cancer. We show that knocking down NGF or its receptors, TRKA and p75NTR, or treatment with GW441756, a TRKA kinase inhibitor, reduces the proliferation and migration of pancreatic cancer cells in vitro. Furthermore, pancreatic cancer cells migrate towards dorsal root ganglia (DRG) in a co-culture assay, indicating a paracrine NGF signaling between the DRGs and pancreatic cancer cells. Knocking down the expression of NGF pathway proteins or inhibiting the activity of TRKA by GW441756 reduced the migratory ability of Mia PaCa2 towards the DRGs. Finally, blocking NGF signaling by NGF neutralizing antibodies or GW441756 inhibited the neurite formation in PC-12 cells in response to conditioned media from pancreatic cancer cells, indicating a reciprocal signaling pathway between the pancreatic cancer cells and nerves. Our results indicate that NGF signaling pathway provides a potential target for developing molecularly targeted therapies to decrease PNI and reduce pain generation. Since there are several TRKA antagonists currently in early clinical trials they could now be tested in the clinical situation of pancreatic cancer induced pain.
Induction of neurite outgrowth in PC12 cells treated with temperature-controlled repeated thermal stimulation.[Pubmed:25879210]
PLoS One. 2015 Apr 16;10(4):e0124024.
To promote the functional restoration of the nervous system following injury, it is necessary to provide optimal extracellular signals that can induce neuronal regenerative activities, particularly neurite formation. This study aimed to examine the regulation of neuritogenesis by temperature-controlled repeated thermal stimulation (TRTS) in rat PC12 pheochromocytoma cells, which can be induced by neurotrophic factors to differentiate into neuron-like cells with elongated neurites. A heating plate was used to apply thermal stimulation, and the correlation of culture medium temperature with varying surface temperature of the heating plate was monitored. Plated PC12 cells were exposed to TRTS at two different temperatures via heating plate (preset surface temperature of the heating plate, 39.5 degrees C or 42 degrees C) in growth or differentiating medium for up to 18 h per day. We then measured the extent of growth, neuritogenesis, or acetylcholine esterase (AChE) activity (a neuronal marker). To analyze the mechanisms underlying the effects of TRTS on these cells, we examined changes in intracellular signaling using the following: tropomyosin-related kinase A inhibitor GW441756; p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580; and MAPK/extracellular signal-regulated kinase (ERK) kinase (MEK) inhibitor U0126 with its inactive analog, U0124, as a control. While a TRTS of 39.5 degrees C did not decrease the growth rate of cells in the cell growth assay, it did increase the number of neurite-bearing PC12 cells and AChE activity without the addition of other neuritogenesis inducers. Furthermore, U0126, and SB203580, but not U0124 and GW441756, considerably inhibited TRTS-induced neuritogenesis. These results suggest that TRTS can induce neuritogenesis and that participation of both the ERK1/2 and p38 MAPK signaling pathways is required for TRTS-dependent neuritogenesis in PC12 cells. Thus, TRTS may be an effective technique for regenerative neuromedicine.
Syntheses and evaluation of carbon-11- and fluorine-18-radiolabeled pan-tropomyosin receptor kinase (Trk) inhibitors: exploration of the 4-aza-2-oxindole scaffold as Trk PET imaging agents.[Pubmed:25350780]
ACS Chem Neurosci. 2015 Feb 18;6(2):260-76.
Tropomyosin receptor kinases (TrkA/B/C) are critically involved in the development of the nervous system, in neurological disorders as well as in multiple neoplasms of both neural and non-neural origins. The development of Trk radiopharmaceuticals would offer unique opportunities toward a more complete understanding of this emerging therapeutic target. To that end, we first developed [(11)C]GW441756 ([(11)C]9), a high affinity photoisomerizable pan-Trk inhibitor, as a lead radiotracer for our positron emission tomography (PET) program. Efficient carbon-11 radiolabeling afforded [(11)C]9 in high radiochemical yields (isolated RCY, 25.9% +/- 5.7%). In vitro autoradiographic studies in rat brain and TrkB-expressing human neuroblastoma cryosections confirmed that [(11)C]9 specifically binds to Trk receptors in vitro. MicroPET studies revealed that binding of [(11)C]9 in the rodent brain was mostly nonspecific despite initial high brain uptake (SUVmax = 2.0). Modeling studies of the 4-aza-2-oxindole scaffold led to the successful identification of a small series of high affinity fluorinated and methoxy derivatized pan-Trk inhibitors based on our lead compound 9. Out of this series, the fluorinated compound 10 was selected for initial evaluation and radiolabeled with fluorine-18 (isolated RCY, 2.5% +/- 0.6%). Compound [(18)F]10 demonstrated excellent Trk selectivity in a panel of cancer relevant kinase targets and a promising in vitro profile in tumors and brain sections but high oxidative metabolic susceptibility leading to nonspecific brain distribution in vivo. The information gained in this study will guide further exploration of the 4-aza-2-oxindole scaffold as a lead for Trk PET ligand development.
The TrkAIII oncoprotein inhibits mitochondrial free radical ROS-induced death of SH-SY5Y neuroblastoma cells by augmenting SOD2 expression and activity at the mitochondria, within the context of a tumour stem cell-like phenotype.[Pubmed:24736663]
PLoS One. 2014 Apr 15;9(4):e94568.
The developmental and stress-regulated alternative TrkAIII splice variant of the NGF receptor TrkA is expressed by advanced stage human neuroblastomas (NBs), correlates with worse outcome in high TrkA expressing unfavourable tumours and exhibits oncogenic activity in NB models. In the present study, we report that constitutive TrkAIII expression in human SH-SY5Y NB cells inhibits Rotenone, Paraquat and LY83583-induced mitochondrial free radical reactive oxygen species (ROS)-mediated death by stimulating SOD2 expression, increasing mitochondrial SOD2 activity and attenuating mitochondrial free radical ROS production, in association with increased mitochondrial capacity to produce H2O2, within the context of a more tumour stem cell-like phenotype. This effect can be reversed by the specific TrkA tyrosine kinase inhibitor GW441756, by the multi-kinase TrkA inhibitors K252a, CEP-701 and Go6976, which inhibit SOD2 expression, and by siRNA knockdown of SOD2 expression, which restores the sensitivity of TrkAIII expressing SH-SY5Y cells to Rotenone, Paraquat and LY83583-induced mitochondrial free radical ROS production and ROS-mediated death. The data implicate the novel TrkAIII/SOD2 axis in promoting NB resistance to mitochondrial free radical-mediated death and staminality, and suggest that the combined use of TrkAIII and/or SOD2 inhibitors together with agents that induce mitochondrial free radical ROS-mediated death could provide a therapeutic advantage that may also target the stem cell niche in high TrkA expressing unfavourable NB.
Paradoxical effect of TrkA inhibition in Alzheimer's disease models.[Pubmed:24531152]
J Alzheimers Dis. 2014;40(3):605-617.
An unbiased screen for compounds that block amyloid-beta protein precursor (AbetaPP) caspase cleavage identified ADDN-1351, which reduced AbetaPP-C31 by 90%. Target identification studies showed that ADDN-1351 is a TrkA inhibitor, and, in complementary studies, TrkA overexpression increased AbetaPP-C31 and cell death. TrkA was shown to interact with AbetaPP and suppress AbetaPP-mediated transcriptional activation. Moreover, treatment of PDAPP transgenic mice with the known TrkA inhibitor GW441756 increased sAbetaPPalpha and the sAbetaPPalpha to Abeta ratio. These results suggest TrkA inhibition-rather than NGF activation-as a novel therapeutic approach, and raise the possibility that such an approach may counteract the hyperactive signaling resulting from the accumulation of active NGF-TrkA complexes due to reduced retrograde transport. The results also suggest that one component of an optimal therapy for Alzheimer's disease may be a TrkA inhibitor.
Proteomic analysis of novel targets associated with TrkA-mediated tyrosine phosphorylation signaling pathways in SK-N-MC neuroblastoma cells.[Pubmed:23319303]
Proteomics. 2013 Jan;13(2):355-67.
Tropomyosin-related kinase A (TrkA) is a receptor-type protein tyrosine kinase and exploits pleiotypic roles via nerve growth factor (NGF)-dependent or NGF-independent mechanisms in various cell types. Here, we showed that the inhibition of TrkA activity by GW441756 resulted in the suppression of tyrosine phosphorylation of cellular proteins including extracellular signal-regulated protein kinase (ERK) and c-Jun N-terminal kinase (JNK). To find novel targets associated with TrkA-mediated tyrosine phosphorylation signaling pathways, we investigated GW441756 effects on TrkA-dependent targets in SK-N-MC neuroblastoma cells by proteomic analysis. The major TrkA-dependent protein spots controlled by GW441756 were determined by PDQuest image analysis, identified by MALDI-TOF MS and MALDI-TOF/TOF MS/MS, and verified by 2DE/Western blot analysis. Thus, we found that most of the identified protein spots were modified forms in a normal condition, and their modifications were regulated by TrkA activity. Especially, our results demonstrated that the modifications of alpha-tubulin and heterogeneous nuclear ribonucleoproteins C1/C2 (hnRNP C1/C2) were significantly upregulated by TrkA, whereas alpha-enolase modification was downregulated by TrkA, and it was suppressed by GW441756, indicating that TrkA activity is required for their modifications. Taken together, we suggest here that the major novel TrkA-dependent targets such as alpha-tubulin, hnRNP C1/C2, and alpha-enolase could play an essential role in TrkA-mediated tyrosine phosphorylation signaling pathways via regulation of their posttranslational modifications.
Screening for novel LRRK2 inhibitors using a high-throughput TR-FRET cellular assay for LRRK2 Ser935 phosphorylation.[Pubmed:22952710]
PLoS One. 2012;7(8):e43580.
BACKGROUND: Mutations in the leucine-rich repeat kinase-2 (LRRK2) have been linked to Parkinson's disease. Recent studies show that inhibition of LRRK2 kinase activity decreased the level of phosphorylation at its own Ser910 and Ser935, indicating that these sites are prime targets for cellular readouts of LRRK2 inhibition. METHODOLOGY/PRINCIPAL FINDINGS: Using Time-Resolved Forster Resonance Energy Transfer (TR-FRET) technology, we developed a high-throughput cellular assay for monitoring LRRK2 phosphorylation at Ser935. LRRK2-Green Fluorescence Protein (GFP) fusions were expressed in cells via BacMam. Phosphorylation at Ser935 in these cells is detected using a terbium labeled anti-phospho-Ser935 antibody that generates a TR-FRET signal between terbium and GFP. LRRK2 wild-type and G2019S are constitutively phosphorylated at Ser935 in cells as measured by TR-FRET. The phosphorylation level is reduced for the R1441C mutant and little could be detected for the kinase-dead mutant D1994A. The TR-FRET cellular assay was further validated using reported LRRK2 inhibitors including LRRK2-IN-1 and our results confirmed that inhibition of LRRK2 can reduce the phosphorylation level at Ser935. To demonstrate the utility of this assay for screening, we profiled a small library of 1120 compounds. Three known LRRK2 inhibitors were identified and 16 hits were followed up in the TR-FRET and a cytotoxicity assay. Interestingly, out of the top 16 hits, five are known inhibitors of IkappaB phosphorylation, two CHK1 and two CDC25 inhibitors. Thirteen hits were further tested in a biochemical LRRK2 kinase activity assay and Western blot analysis for their effects on the phosphorylation of Ser910, Ser935, Ser955 and Ser973. CONCLUSIONS/SIGNIFICANCE: We developed a TR-FRET cellular assay for LRRK2 Ser935 phosphorylation that can be applied to the screening for LRRK2 inhibitors. We report for the first time that several compounds such as IKK16, CHK1 inhibitors and GW441756 can inhibit LRRK2 Ser935 phosphorylation in cells and LRRK2 kinase activity in vitro.
Nerve growth factor induces the expression of chaperone protein calreticulin in human epithelial ovarian cells.[Pubmed:22773372]
Horm Metab Res. 2012 Jul;44(8):639-43.
Epithelial ovarian cancer is highly angiogenic and high expression of Nerve Growth Factor (NGF), a proangiogenic protein. Calreticulin is a multifunctional protein with anti-angiogenic properties and its translocation to the tumor cell membrane promotes recognition and engulfment by dendritic cells. The aim of this work was to evaluate calreticulin expression in human normal ovaries, benign and borderline tumors, and epithelial ovarian cancer samples and to evaluate whether NGF regulates calreticulin expression in human ovarian surface epithelium and in epithelial ovarian cancer cell lines. Calreticulin mRNA and protein levels were analyzed using RT-PCR, Western blot and immunohistochemistry in 67 human ovarian samples obtained from our Institution. Calreticulin expression induced by NGF stimulation in cell lines was evaluated using RT-PCR, Western blot and immunocytochemistry. We found a significant increase of calreticulin mRNA levels in epithelial ovarian cancer samples as compared to normal ovaries, benign tumors, and borderline tumors. Calreticulin protein levels, evaluated by Western blot, were also increased in epithelial ovarian cancer with respect to benign and borderline tumors. When HOSE and A2780 cell lines were stimulated with Nerve Growth Factor, we found an increase in calreticulin protein levels compared to controls. This effect was reverted by GW441756, a TRKA specific inhibitor. These results suggest that NGF regulates calreticulin protein levels in epithelial ovarian cells through TRKA receptor activation.
Dorsomorphin stimulates neurite outgrowth in PC12 cells via activation of a protein kinase A-dependent MEK-ERK1/2 signaling pathway.[Pubmed:21988724]
Genes Cells. 2011 Nov;16(11):1121-32.
In this study, we investigated the effect of dorsomorphin, a selective inhibitor of bone morphogenetic protein (BMP) signaling, on rat PC12 pheochromocytoma cell differentiation. PC12 cells can be induced to differentiate into neuron-like cells possessing elongated neurites by nerve growth factor, BMP2, and other inducers. Cells were incubated with BMP2 and/or dorsomorphin, and the extent of neurite outgrowth was evaluated. Unexpectedly, BMP2-mediated neuritogenesis was not inhibited by co-treatment with dorsomorphin. We also found that treatment with dorsomorphin alone, but not another BMP signaling inhibitor, LDN-193189, induced neurite outgrowth in PC12 cells. To further understand the mechanism of action of dorsomorphin, the effects of this drug on intracellular signaling were investigated using the following signaling inhibitors: the ERK kinase (MEK) inhibitor U0126; the tropomyosin-related kinase A inhibitor GW441756; and the protein kinase A (PKA) inhibitor H89. Dorsomorphin induced rapid and sustained ERK1/2 activation; however, dorsomorphin-mediated ERK1/2 activation and neuritogenesis were robustly inhibited in the presence of U0126 or H89, but not GW441756. These findings suggest that dorsomorphin has the potential to induce neuritogenesis in PC12 cells, a response that requires the activation of PKA-dependent MEK-ERK1/2 signaling.
Cytosolic accumulation of gammaH2AX is associated with tropomyosin-related kinase A-induced cell death in U2OS cells.[Pubmed:18587265]
Exp Mol Med. 2008 Jun 30;40(3):276-85.
Tropomyosin-related kinase A (TrkA) plays an important role in cell survival, differentiation, and apoptosis in various neuronal and nonneuronal cell types. Here we show that TrkA overexpression by the Tet-On system mimics NGF-mediated activation pathways in the absence of nerve growth factor (NGF) stimulation in U2OS cells. In addition, p53 upregulation upon DNA damage was inhibited by TrkA, and p21 was upregulated by TrkA in a p53-independent manner. TrkA overexpression caused cell death by interrupting cell cycle progression, and TrkA-induced cell death was diminished in the presence of its specific inhibitor GW441756. Interestingly, TrkA-mediated cell death was strongly related to gammaH2AX production and poly (ADP-ribose) polymerase cleavage in the absence of DNA damage inducer. In this study, we also reveal that gammaH2AX production by TrkA is blocked by TrkA kinase inhibitors K-252a and GW441756, and it is also significantly inhibited by JNK inhibitor SP600125. Moreover, reduction of cell viability by TrkA was strongly suppressed by SP600125 treatment, suggesting a critical role of JNK in TrkA-induced cell death. We also found that gammaH2AX and TrkA were colocalized in cytosol in the absence of DNA damage, and the nuclear localization of gammaH2AX induced by DNA damage was partly altered to cytosol by TrkA overexpression. Our results suggest that the abnormal cytosolic accumulation of gammaH2AX is implicated in TrkA-induced cell death in the absence of DNA damage.
Apoptotic cell death in TrkA-overexpressing cells: kinetic regulation of ERK phosphorylation and caspase-7 activation.[Pubmed:18511888]
Mol Cells. 2008 Jul 31;26(1):12-7. Epub 2008 May 20.
The TrkA tyrosine kinase is activated by autophosphorylation in response to NGF, and plays an important role in cell survival, differentiation, and apoptosis. To investigate its role in cell fate determination, we produced stable TrkA-inducible SK-N-MC and U2OS cell lines using the Tet-On system. Interestingly, TrkA overexpression induced substantial cell death even in the absence of NGF, by stimulating ERK phosphorylation and caspase-7 activation leading to PARP cleavage. TrkA-mediated cell death was shown by the annexin-V binding assay to be, at least in part, apoptotic in both SK-N-MC and U2OS cells. Furthermore, the truncated form (p18) of Bax accumulated in the TrkA-induced cells, suggesting that TrkA induces mitochondria-mediated apoptosis. NGF treatment augmented the cell death induced by TrkA overexpression. This TrkA-induced cell death was blocked by the tyrosine kinase inhibitors, K-252a and GW441756. Moreover, TrkA overexpression inhibited long-term proliferation of both the neuronal SK-N-MC cells and the non-neuronal U2OS cells, suggesting a potential role of TrkA as a tumor suppressor.
Discovery and in vitro evaluation of potent TrkA kinase inhibitors: oxindole and aza-oxindoles.[Pubmed:15013000]
Bioorg Med Chem Lett. 2004 Feb 23;14(4):953-7.
The discovery, synthesis, potential binding mode, and in vitro kinase profile of 3-(3-bromo-4-hydroxy-5-(2'-methoxyphenyl)-benzylidene)-5-bromo-1,3-dihydro-pyrrol o[2,3-b]pyridin-2-one, 3-[(1-methyl-1H-indol-3-yl)methylene]-1,3-dihydro-2H-pyrrolo[3,2-b]-pyridin-2-one as potent TrkA inhibitors are discussed.


