HomobaldrinalCAS# 67910-07-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 67910-07-0 | SDF | Download SDF |
| PubChem ID | 49999 | Appearance | Powder |
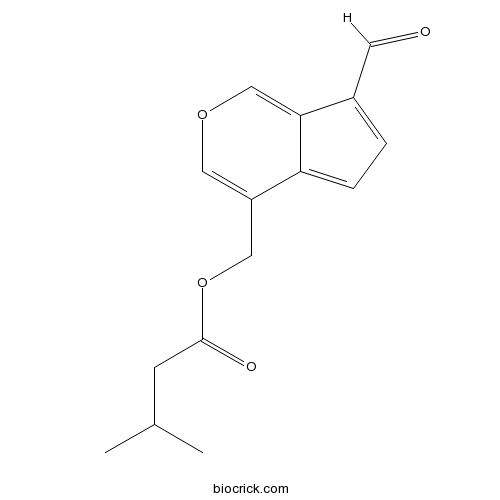
| Formula | C15H16O4 | M.Wt | 260.28 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (7-formylcyclopenta[c]pyran-4-yl)methyl 3-methylbutanoate | ||
| SMILES | CC(C)CC(=O)OCC1=COC=C2C1=CC=C2C=O | ||
| Standard InChIKey | LTVSLKWNAZKBEX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H16O4/c1-10(2)5-15(17)19-8-12-7-18-9-14-11(6-16)3-4-13(12)14/h3-4,6-7,9-10H,5,8H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Homobaldrinal has low toxic activity. 2. Homobaldrinal has genotoxic activity in the Salmonella/microsome test and the SOS-chromotest. |

Homobaldrinal Dilution Calculator

Homobaldrinal Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.842 mL | 19.2101 mL | 38.4202 mL | 76.8403 mL | 96.0504 mL |
| 5 mM | 0.7684 mL | 3.842 mL | 7.684 mL | 15.3681 mL | 19.2101 mL |
| 10 mM | 0.3842 mL | 1.921 mL | 3.842 mL | 7.684 mL | 9.605 mL |
| 50 mM | 0.0768 mL | 0.3842 mL | 0.7684 mL | 1.5368 mL | 1.921 mL |
| 100 mM | 0.0384 mL | 0.1921 mL | 0.3842 mL | 0.7684 mL | 0.9605 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dehydroevodiamine
Catalog No.:BCN2974
CAS No.:67909-49-3
- Aluminum n-octacosoxide
Catalog No.:BCC8099
CAS No.:67905-27-5
- Ambrox
Catalog No.:BCN6907
CAS No.:6790-58-5
- Scutebarbatine K
Catalog No.:BCN3223
CAS No.:960302-86-7
- Cyclo(Pro-Trp)
Catalog No.:BCN2422
CAS No.:67889-75-2
- Gigantol
Catalog No.:BCN8382
CAS No.:67884-30-4
- n-Butyl-β-D-fructopyranoside
Catalog No.:BCC9097
CAS No.:67884-27-9
- Martynoside
Catalog No.:BCN4236
CAS No.:67884-12-2
- Deapi-platycodin D3
Catalog No.:BCN3240
CAS No.:67884-05-3
- Glycosolone
Catalog No.:BCN6521
CAS No.:67879-81-6
- Caftaric acid
Catalog No.:BCN2096
CAS No.:67879-58-7
- Boc-Nva-OH.DCHA
Catalog No.:BCC2642
CAS No.:67861-96-5
- Macusine B
Catalog No.:BCN6471
CAS No.:6792-07-0
- Mesaconine
Catalog No.:BCC8339
CAS No.:6792-09-2
- 1,6-Dihydro-4,7'-epoxy-1-methoxy-3',4'-methylenedioxy-6-oxo-3,8'-lignan
Catalog No.:BCN6584
CAS No.:67920-48-3
- Sodium Danshensu
Catalog No.:BCN5952
CAS No.:67920-52-9
- Aurantio-obtusin
Catalog No.:BCN1222
CAS No.:67979-25-3
- N-(5,8,11,14-Eicosatetraenoyl)taurine
Catalog No.:BCN1755
CAS No.:679834-28-7
- N-(15-Methyl-9-hexadecenoyl)taurine
Catalog No.:BCN1754
CAS No.:679834-30-1
- BMS 193885
Catalog No.:BCC7613
CAS No.:679839-66-8
- Sodium citrate
Catalog No.:BCC7588
CAS No.:68-04-2
- Vitamin B12
Catalog No.:BCC4878
CAS No.:68-19-9
- Norethindrone
Catalog No.:BCC4811
CAS No.:68-22-4
- Vitamin A
Catalog No.:BCN8349
CAS No.:68-26-8
Cytotoxic potential of valerian constituents and valerian tinctures.[Pubmed:23195845]
Phytomedicine. 1998 May;5(3):219-25.
Underground parts of three Valeriana species, namely V. officinalis L. s.l., V. wallichii DC. (V. jatamansi Jones), and V. edulis Nutt. ex Torr & Gray ssp. procera (H.B.K.) F. G. Meyer (V. mexicana DC.), are used in phytotherapy because of their mild sedative properties. Characteristic constituents of these species, which are regarded also as the active principles, were tested for cytotoxicity against GLC(4), a human small-cell lung cancer cell line, and against COLO 320, a human colorectal cancer cell line, using the microculture tetrazolium (MTT) assay. Valepotriates of the diene type (valtrate, isovaltrate and acevaltrate) displayed the highest cytotoxicity, with IC50 values of 1-6 muM, following continuous incubation. The monoene type valepotriates (didrovaltrate and isovaleroxyhydroxydidrovaltrate) were 2- to 3-fold less toxic. Baldrinal and Homobaldrinal, decomposition products of valepotriates, were 10- to 30-fold less toxic than their parent compounds. Isovaltral had a higher cytotoxicity than its parent compound isovaltrate. Valerenic acids (valerenic acid, acetoxyvalerenic acid, hydroxyvalerenic acid and methyl valerenate), which are characteristic for V. officinalis, had a low toxicity with IC(50) values between 100 and 200 muM. Freshly prepared and stored tinctures, prepared from roots and rhizomes of the three valerian species, were analysed for valepotriates, baldrinals and valerenic acids, and also tested for cytotoxicity. There was a clear relationship between the valepotriate contents of the freshly prepared tinctures and their toxicity. Upon storage, valepotriates decomposed, which was reflected in a significant reduction of the cytotoxic effect.
Bacterial mutagenicity of the tranquilizing constituents of Valerianaceae roots.[Pubmed:3511364]
Mutat Res. 1986 Jan-Feb;169(1-2):23-7.
The valepotriates valtrate/isovaltrate and dihydrovaltrate are considered to be the main tranquilizing constituents of drugs derived from the roots of several Valerianaceae. The decomposition products of valtrate and isovaltrate include the metabolites baldrinal and Homobaldrinal, respectively, whereas the decomposition products of dihydrovaltrate do not include baldrinal-like metabolites. Purified valtrate/isovaltrate, dihydrovaltrate, baldrinal and Homobaldrinal were investigated for their genotoxic activity in the Salmonella/microsome test and the SOS-chromotest. The valepotriates developed mutagenic activity in these test systems only in the presence of S9 mix, whereas both baldrinals showed mutagenic effects in both tests with and without metabolic activation.


