Hemiphroside ACAS# 165338-27-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 165338-27-2 | SDF | Download SDF |
| PubChem ID | 91884993 | Appearance | Powder |
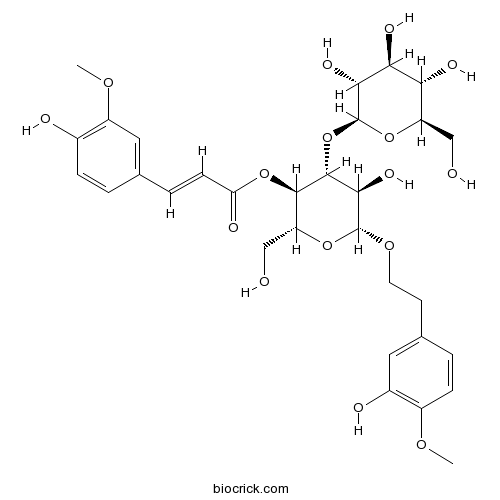
| Formula | C31H40O16 | M.Wt | 668.7 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(2R,3R,4R,5R,6R)-5-hydroxy-6-[2-(3-hydroxy-4-methoxyphenyl)ethoxy]-2-(hydroxymethyl)-4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-3-yl] (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate | ||
| SMILES | COC1=C(C=C(C=C1)CCOC2C(C(C(C(O2)CO)OC(=O)C=CC3=CC(=C(C=C3)O)OC)OC4C(C(C(C(O4)CO)O)O)O)O)O | ||
| Standard InChIKey | VKUXVDUAEAQCSK-MJLPRLLOSA-N | ||
| Standard InChI | InChI=1S/C31H40O16/c1-41-19-7-4-16(11-18(19)35)9-10-43-30-27(40)29(47-31-26(39)25(38)24(37)21(13-32)44-31)28(22(14-33)45-30)46-23(36)8-5-15-3-6-17(34)20(12-15)42-2/h3-8,11-12,21-22,24-35,37-40H,9-10,13-14H2,1-2H3/b8-5+/t21-,22-,24-,25+,26-,27-,28-,29-,30-,31+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Hemiphroside A is a natural product from Hydrangea macrophylla. |
| In vitro | Antioxidative phenylethanoid and phenolic glycosides from Picrorhiza scrophulariiflora.[Pubmed: 15133218]Chem Pharm Bull (Tokyo). 2004 May;52(5):615-7.
|
| Structure Identification | Zhongguo Zhong Yao Za Zhi. 2004 Jun;29(6):531-4.[Phenylethanoid glycosides from root of Picrorhiza scrophulariiflora].[Pubmed: 15706916]To study the phenylethanoid glycosides from root of Picrorhiza scrophulariiflora. |

Hemiphroside A Dilution Calculator

Hemiphroside A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4954 mL | 7.4772 mL | 14.9544 mL | 29.9088 mL | 37.386 mL |
| 5 mM | 0.2991 mL | 1.4954 mL | 2.9909 mL | 5.9818 mL | 7.4772 mL |
| 10 mM | 0.1495 mL | 0.7477 mL | 1.4954 mL | 2.9909 mL | 3.7386 mL |
| 50 mM | 0.0299 mL | 0.1495 mL | 0.2991 mL | 0.5982 mL | 0.7477 mL |
| 100 mM | 0.015 mL | 0.0748 mL | 0.1495 mL | 0.2991 mL | 0.3739 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-Ethyl-3-nitrophthalate
Catalog No.:BCC8466
CAS No.:16533-45-2
- Cleroindicin E
Catalog No.:BCN1729
CAS No.:165197-71-7
- Shizukaol G
Catalog No.:BCN7879
CAS No.:165171-11-9
- Shizukaol E
Catalog No.:BCN7880
CAS No.:165171-09-5
- 6-Epidemethylesquirolin D
Catalog No.:BCN1728
CAS No.:165074-00-0
- Brevilin A
Catalog No.:BCN1727
CAS No.:16503-32-5
- Nα-Methylhistamine dihydrochloride
Catalog No.:BCC6701
CAS No.:16503-22-3
- Segetalin B
Catalog No.:BCC9247
CAS No.:164991-89-3
- Orcinol gentiobioside
Catalog No.:BCN2815
CAS No.:164991-86-0
- Decumbenine B
Catalog No.:BCC8313
CAS No.:164991-68-8
- Kukoamine B
Catalog No.:BCN3836
CAS No.:164991-67-7
- Calyxin B
Catalog No.:BCN1726
CAS No.:164991-53-1
- Hemiphroside B
Catalog No.:BCN1731
CAS No.:165338-28-3
- SA 4503 dihydrochloride
Catalog No.:BCC6339
CAS No.:165377-44-6
- bis[6-(5,6-dihydrochelerythrinyl)]amine
Catalog No.:BCN8232
CAS No.:165393-48-6
- 16-Hydroxy-2-oxocleroda-3,13-dien-15,16-olide
Catalog No.:BCN1536
CAS No.:165459-53-0
- Di-Dnp-L-Lysine
Catalog No.:BCC8939
CAS No.:1655-49-8
- DEPBT
Catalog No.:BCC2811
CAS No.:165534-43-0
- 12-O-tetradecanoylphorbol-13-acetate
Catalog No.:BCN2511
CAS No.:16561-29-8
- L-Stepholidine
Catalog No.:BCN2599
CAS No.:16562-13-3
- Methyl 6-acetoxyangolensate
Catalog No.:BCN1732
CAS No.:16566-88-4
- Torososide A
Catalog No.:BCN4694
CAS No.:165689-32-7
- Linezolid
Catalog No.:BCC2496
CAS No.:165800-03-3
- Eperezolid
Catalog No.:BCC5177
CAS No.:165800-04-4
[Phenylethanoid glycosides from root of Picrorhiza scrophulariiflora].[Pubmed:15706916]
Zhongguo Zhong Yao Za Zhi. 2004 Jun;29(6):531-4.
OBJECTIVE: To study the phenylethanoid glycosides from root of Picrorhiza scrophulariiflora. METHOD: Column chromatographic techniques were used for isolation and purification of chemical constituents of the plant and the structures were identified by spectroscopic analysis. RESULT: Six phenylethanoid glycosides were isolated and elucidated as: 2-(3,4-dihydroxyphenyl)-ethyl-O-beta-D-glucopyranoside (1), 2-(3-hydroxy-4-methoxyphenyl)-ethyl-O-beta-D-glucopyranosyl (1-->3) beta-D-glucopyranoside (2), scroside B (3), Hemiphroside A (4), plantainoside D (5) and scroside A (6), respectively. CONCLUSION: Compounds 1, 2, 4 and 5 were isolated from this plant for the first time and compound 2 was firstly obtained from natural source.
Antioxidative phenylethanoid and phenolic glycosides from Picrorhiza scrophulariiflora.[Pubmed:15133218]
Chem Pharm Bull (Tokyo). 2004 May;52(5):615-7.
One new phenylenthanoid glycoside, scroside D (2), was isolated from the roots of Picrorhiza scrophulariiflora (Scrophulariaceae), together with nine known phenylethanoid and phenolic glycosides: 2-(3,4-dihydroxyphenyl)-ethyl-O-beta-D-glucopyranoside (1), 2-(3-hydroxy-4-methoxyphenyl)-ethyl-O-beta-D-glucopyranosyl (1-->3)-beta-D-glucopyranoside (3), scroside B (4), Hemiphroside A (5), plantainoside D (6), scroside A (7), androsin (8), piceoside (9), and 6-O-feruloyl-beta-D-glucopyranoside (10). The structures of these compounds were elucidated using spectroscopic methods. The antioxidative activities of these isolated compounds were evaluated based on their scavenging effects on hydroxyl radicals and superoxide anion radicals, respectively. Compounds 1, 2, and 6 showed potent antioxidative effects as those of ascorbic acid and the structure-activity relationship is discussed.
[Studies on chemical constituents from herb of Hemiphragma heterophyllum].[Pubmed:15706915]
Zhongguo Zhong Yao Za Zhi. 2004 Jun;29(6):528-31.
OBJECTIVE: To study the chemical constituents from Hemiphragma heterophyllum. METHOD: The constituents were isolated and purified by chromatographic technology and their structures were elucidated by physico-chemical evidence and spectral data. RESULT: Eight compounds were isolated and identified as hemiphroside C (I), plantainoside E (II), Hemiphroside A (III), acetoside (IV), globularin (V), 10-(Z)-cinnamoyl-catapol (VI), iso-scrophularioside (VII), cinnamic acid (VIII). CONCLUSION: Compound I was a new phenylethanoid glucoside and compounds II and VI were isolated for the first time from this plant.


