HelicidCAS# 80154-34-3 |
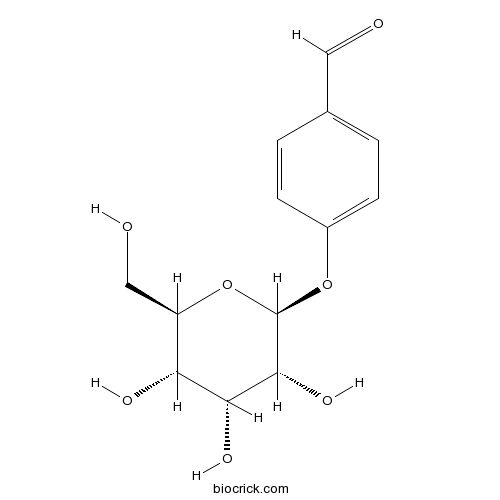
- p-Hydroxybenzaldehyde glucoside
Catalog No.:BCN9400
CAS No.:26993-16-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 80154-34-3 | SDF | Download SDF |
| PubChem ID | 12896796 | Appearance | White powder |
| Formula | C13H16O7 | M.Wt | 284.26 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | Helicide | ||
| Solubility | Soluble in methanol and pyridine | ||
| Chemical Name | 4-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxybenzaldehyde | ||
| SMILES | C1=CC(=CC=C1C=O)OC2C(C(C(C(O2)CO)O)O)O | ||
| Standard InChIKey | OLZAGZCCJJBKNZ-SYLRKERUSA-N | ||
| Standard InChI | InChI=1S/C13H16O7/c14-5-7-1-3-8(4-2-7)19-13-12(18)11(17)10(16)9(6-15)20-13/h1-5,9-13,15-18H,6H2/t9-,10-,11-,12-,13-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Helicid analogues are mushroom tyrosinase inhibitors, some of them have more potent inhibitory activities than arbutin (IC50 =7.3 mM).Some helicid analogues exhibit potent cholinesterase (AChE) inhibitory activities. |
| Targets | AChR |
| In vitro | Bioavailability and pharmacokinetics profile of helicid in beagle dogs using gradient elution high performance liquid chromatography electrospray ionization mass spectrometry.[Pubmed: 25743699]J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Apr 15;988:8-12.A simple, sensitive and reliable gradient elution high performance liquid chromatography electrospray ionization mass spectrometry (HPLC-ESI-MS) method was developed for quantifying Helicid in dog plasma. |
| Kinase Assay | Synthesis and biological evaluation of helicid analogues as novel acetylcholinesterase inhibitors.[Pubmed: 17574306 ]Synthesis and biological evaluation of helicid analogues as mushroom tyrosinase inhibitors.[Pubmed: 18996693 ]Bioorg Med Chem Lett. 2008 Dec 15;18(24):6490-3.
Eur J Med Chem. 2008 Jan;43(1):166-73.
|
| Structure Identification | Spectrochim Acta A Mol Biomol Spectrosc. 2014 Apr 24;124:46-51.Binding of helicid to human serum albumin: a hybrid spectroscopic approach and conformational study.[Pubmed: 24463239]
|

Helicid Dilution Calculator

Helicid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5179 mL | 17.5895 mL | 35.1791 mL | 70.3581 mL | 87.9477 mL |
| 5 mM | 0.7036 mL | 3.5179 mL | 7.0358 mL | 14.0716 mL | 17.5895 mL |
| 10 mM | 0.3518 mL | 1.759 mL | 3.5179 mL | 7.0358 mL | 8.7948 mL |
| 50 mM | 0.0704 mL | 0.3518 mL | 0.7036 mL | 1.4072 mL | 1.759 mL |
| 100 mM | 0.0352 mL | 0.1759 mL | 0.3518 mL | 0.7036 mL | 0.8795 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methyl demethoxycarbonylchanofruticosinate
Catalog No.:BCN1348
CAS No.:80151-89-9
- Tussilagine
Catalog No.:BCN1984
CAS No.:80151-77-5
- L-Alanosine
Catalog No.:BCN7253
CAS No.:5854-93-3
- GSK256066
Catalog No.:BCC2285
CAS No.:801312-28-7
- YC 170
Catalog No.:BCC1212
CAS No.:59946-73-5
- H-D-Phe(3-Cl)-OH
Catalog No.:BCC3167
CAS No.:80126-52-9
- H-Phe(3-Cl)-OH
Catalog No.:BCC3168
CAS No.:80126-51-8
- H-D-Phe(2-Cl)-OH
Catalog No.:BCC3166
CAS No.:80126-50-7
- Boc-D-Phe(3-Cl)-OH
Catalog No.:BCC3169
CAS No.:80102-25-6
- Agatharesinol acetonide
Catalog No.:BCN4574
CAS No.:800389-33-7
- Soybean phospholipid
Catalog No.:BCN3888
CAS No.:8002-43-5
- Dihydrocholesterol
Catalog No.:BCN2749
CAS No.:80-97-7
- Sorbic acid, 1-p-tolylhydrazide
Catalog No.:BCN2219
CAS No.:802048-02-8
- Roxithromycin
Catalog No.:BCC4842
CAS No.:80214-83-1
- Glochidionionol C
Catalog No.:BCC2641
CAS No.:
- Rosmanol
Catalog No.:BCN8425
CAS No.:80225-53-2
- 2'-O-Galloylquercitrin
Catalog No.:BCN8225
CAS No.:80229-08-9
- Casanthranol
Catalog No.:BCC3746
CAS No.:8024-48-4
- Spiramycin
Catalog No.:BCC4724
CAS No.:8025-81-8
- Nafamostat hydrochloride
Catalog No.:BCC4188
CAS No.:80251-32-7
- PHA-848125
Catalog No.:BCC3839
CAS No.:802539-81-7
- Artemisinic acid
Catalog No.:BCN4336
CAS No.:80286-58-4
- AZD1981
Catalog No.:BCC4506
CAS No.:802904-66-1
- RG7090
Catalog No.:BCC5499
CAS No.:802906-73-6
Bioavailability and pharmacokinetics profile of helicid in beagle dogs using gradient elution high performance liquid chromatography electrospray ionization mass spectrometry.[Pubmed:25743699]
J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Apr 15;988:8-12.
A simple, sensitive and reliable gradient elution high performance liquid chromatography electrospray ionization mass spectrometry (HPLC-ESI-MS) method was developed for quantifying Helicid in dog plasma. The limit of detection (LOD) and the lower limit of quantitation (LLOQ) were 0.3 and 1 ng/mL, respectively. This method was validated for selectivity, linearity, accuracy and precision, extraction recoveries, matrix effects, carry-over, cross-talk, dilution integrity, stability and incurred sample reanalysis (ISR). Bioavailability and pharmacokinetic parameters of Helicid in beagle dogs were researched from a two period crossover design study. After intravenous administration (i.v.), Helicid had a mean (+/- SD) AUC0-infinity of 12062.06 +/- 2482.69 ng/mL h and terminal half-life (t1/2 z) of 2.91 +/- 1.37 h, while Cmax was 35613.23 +/- 8157.18 ng/mL. Following intragastric gavage administration (i.g.), AUC0-infinity was 7589.16 +/- 1797.20 ng/mL h along with a longer t1/2 z of 4.10 +/- 4.35 h. Cmax was researched at 0.58 +/- 0.20 h. The absolute bioavailability (F) of Helicid was 15.74 +/- 1.87%.
Synthesis and biological evaluation of helicid analogues as novel acetylcholinesterase inhibitors.[Pubmed:17574306]
Eur J Med Chem. 2008 Jan;43(1):166-73.
A series of Helicid analogues were prepared and evaluated in vitro for the cholinesterase (AChE and BuChE) inhibitory activities via UV spectroscopy. The results indicated that compounds 5, 6d and 8 exhibited potent AChE inhibitory activities with IC(50) values of 0.45+/-0.02microM, 0.49+/-0.02microM, and 0.20+/-0.01microM, respectively. High selectivity for AChE over BuChE was also observed. Kinetic study showed that the mechanism of AChE inhibition of compounds 5, 6d and 8 was all mixed-type.
Synthesis and biological evaluation of helicid analogues as mushroom tyrosinase inhibitors.[Pubmed:18996693]
Bioorg Med Chem Lett. 2008 Dec 15;18(24):6490-3.
A series of Helicid analogues were synthesized and evaluated as tyrosinase inhibitors. The results demonstrated that some compounds had more potent inhibitory activities than arbutin (IC(50) 7.3 mM). In particular, compound 1c bearing 4,6-O-benzylidene substituent on the sugar moiety was found to be the most potent inhibitor with IC(50) value of 0.052 mM. The inhibition kinetics analyzed by Lineweaver-Burk plots revealed that Helicid analogues were competitive inhibitors. The Circular dichroism spectra indicated that those compounds induced conformational changes of mushroom tyrosinase upon binding.
Binding of helicid to human serum albumin: a hybrid spectroscopic approach and conformational study.[Pubmed:24463239]
Spectrochim Acta A Mol Biomol Spectrosc. 2014 Apr 24;124:46-51.
The interaction between human serum albumin and Helicid was studied by steady-state fluorescence, ultraviolet-visible, circular dichroism, Fourier transform infrared techniques and molecular modeling. The binding site numbers, association constants, and corresponding thermodynamic parameters were used to investigate the quenching mechanism. The alternations of protein secondary structure in the presence of Helicid were demonstrated using synchronous fluorescence, Fourier transform infrared, circular dichroism and three-dimensional fluorescence spectra. The molecular modeling results revealed that Helicid could bind to hydrophobic pocket of HSA with hydrophobic and hydrogen bond force. The binding site of Helicid in HSA was ascertained. Moreover, an apparent distance of 3.33 nm between the Trp214 and Helicid was obtained via fluorescence resonance energy transfer method.


