Artemisinic acidCAS# 80286-58-4 |
Quality Control & MSDS
Number of papers citing our products

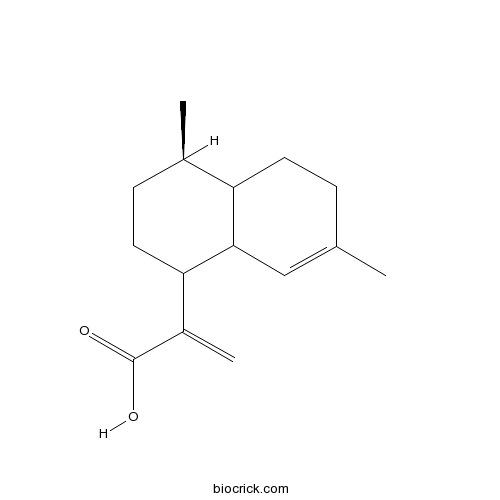
Chemical structure

3D structure
| Cas No. | 80286-58-4 | SDF | Download SDF |
| PubChem ID | 124033 | Appearance | Powder |
| Formula | C15H22O2 | M.Wt | 234.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (426.75 mM; Need ultrasonic) | ||
| Chemical Name | 2-[(4R)-4,7-dimethyl-1,2,3,4,4a,5,6,8a-octahydronaphthalen-1-yl]prop-2-enoic acid | ||
| SMILES | CC1CCC(C2C1CCC(=C2)C)C(=C)C(=O)O | ||
| Standard InChIKey | PLQMEXSCSAIXGB-GOHLOCJQSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Artemisinic acid, is the immediate precursor of the semi-synthesis artemisinin, could be a cost-effective, environmentally friendly, high-quality and reliable source of artemisinin. Artemisinic acid has a variety of pharmacological activity, such as antimalarial, anti-tumor, antipyretic, antibacterial, allelopathy and anti-adipogenesis effects. Artemisinic acid is a regulator of adipocyte differentiation and C/EBP δ expression, it inhibits adipogenic differentiation of hAMSCs through reduced expression of C/EBP δ; it inhibits melanogenesis through downregulation of C/EBP α-dependent expression of HMG-CoA reductase gene. |
| Targets | Tyrosinase | cAMP | PKA | HMG-CoA Reductase | PPAR | JNK | VEGFR | P450 (e.g. CYP17) | Antifection | C/EBP δ |
| In vitro | Artemisinic acid is a regulator of adipocyte differentiation and C/EBP δ expression.[Pubmed: 22396222]J Cell Biochem. 2012 Jul;113(7):2488-99.Adipocyte dysfunction is associated with the development of obesity. In this study, Artemisinic acid, which was isolated from Artemisia annua L., inhibited adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells (hAMSCs) and its mechanism of action was determined.
Antimicrobial activity of artemisinin and its precursors.[Reference: WebLink]Current Science, 2000 , 78 (6) :709-713.Artemisinic acid and Arteannuin B are biogenetic precursors of Artemisinin, an important antimalarial produced by the herb Artemisia annua. These compounds have been screened for antimicrobial activity against a range of organisms.
|
| Kinase Assay | Artemisinic acid inhibits melanogenesis through downregulation of C/EBP α-dependent expression of HMG-CoA reductase gene.[Pubmed: 23063590]Food Chem Toxicol. 2013 Jan;51:225-30.Cholesterol is associated with the regulation of melanogenesis which is the major physiological defense against solar irradiation.
The present study was designed to determine the effects of Artemisinic acid on melanogenesis and its mechanisms of action in human epidermal melanocytes.
|
| Structure Identification | Nature. 2006 Apr 13;440(7086):940-3.Production of the antimalarial drug precursor artemisinic acid in engineered yeast.[Pubmed: 16612385 ]Malaria is a global health problem that threatens 300-500 million people and kills more than one million people annually. Disease control is hampered by the occurrence of multi-drug-resistant strains of the malaria parasite Plasmodium falciparum. Synthetic antimalarial drugs and malarial vaccines are currently being developed, but their efficacy against malaria awaits rigorous clinical testing. Artemisinin, a sesquiterpene lactone endoperoxide extracted from Artemisia annua L (family Asteraceae; commonly known as sweet wormwood), is highly effective against multi-drug-resistant Plasmodium spp., but is in short supply and unaffordable to most malaria sufferers. Although total synthesis of artemisinin is difficult and costly, the semi-synthesis of artemisinin or any derivative from microbially sourced Artemisinic acid, its immediate precursor, could be a cost-effective, environmentally friendly, high-quality and reliable source of artemisinin.
|

Artemisinic acid Dilution Calculator

Artemisinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.268 mL | 21.3402 mL | 42.6803 mL | 85.3606 mL | 106.7008 mL |
| 5 mM | 0.8536 mL | 4.268 mL | 8.5361 mL | 17.0721 mL | 21.3402 mL |
| 10 mM | 0.4268 mL | 2.134 mL | 4.268 mL | 8.5361 mL | 10.6701 mL |
| 50 mM | 0.0854 mL | 0.4268 mL | 0.8536 mL | 1.7072 mL | 2.134 mL |
| 100 mM | 0.0427 mL | 0.2134 mL | 0.4268 mL | 0.8536 mL | 1.067 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PHA-848125
Catalog No.:BCC3839
CAS No.:802539-81-7
- Nafamostat hydrochloride
Catalog No.:BCC4188
CAS No.:80251-32-7
- Spiramycin
Catalog No.:BCC4724
CAS No.:8025-81-8
- Casanthranol
Catalog No.:BCC3746
CAS No.:8024-48-4
- 2'-O-Galloylquercitrin
Catalog No.:BCN8225
CAS No.:80229-08-9
- Rosmanol
Catalog No.:BCN8425
CAS No.:80225-53-2
- Glochidionionol C
Catalog No.:BCC2641
CAS No.:
- Roxithromycin
Catalog No.:BCC4842
CAS No.:80214-83-1
- Sorbic acid, 1-p-tolylhydrazide
Catalog No.:BCN2219
CAS No.:802048-02-8
- Helicid
Catalog No.:BCN1056
CAS No.:80154-34-3
- Methyl demethoxycarbonylchanofruticosinate
Catalog No.:BCN1348
CAS No.:80151-89-9
- Tussilagine
Catalog No.:BCN1984
CAS No.:80151-77-5
- AZD1981
Catalog No.:BCC4506
CAS No.:802904-66-1
- RG7090
Catalog No.:BCC5499
CAS No.:802906-73-6
- Dehydro-δ-tocopherol
Catalog No.:BCN4573
CAS No.:802909-72-4
- Stevenleaf
Catalog No.:BCN5978
CAS No.:80321-63-7
- Gypenoside XVII
Catalog No.:BCN2339
CAS No.:80321-69-3
- 8-Acetonyldihydronitidine
Catalog No.:BCN3304
CAS No.:80330-39-8
- Tinnevellin glucoside
Catalog No.:BCN3414
CAS No.:80358-06-1
- NSC59984
Catalog No.:BCC6540
CAS No.:803647-40-7
- 2',3,5,6',7-Pentahydroxyflavanone
Catalog No.:BCN4337
CAS No.:80366-15-0
- 8beta-Tigloyloxyreynosin
Catalog No.:BCN7222
CAS No.:80368-31-6
- Obatoclax mesylate (GX15-070)
Catalog No.:BCC2234
CAS No.:803712-79-0
- Z-D-Thr-OH
Catalog No.:BCC2736
CAS No.:80384-27-6
Production of the antimalarial drug precursor artemisinic acid in engineered yeast.[Pubmed:16612385]
Nature. 2006 Apr 13;440(7086):940-3.
Malaria is a global health problem that threatens 300-500 million people and kills more than one million people annually. Disease control is hampered by the occurrence of multi-drug-resistant strains of the malaria parasite Plasmodium falciparum. Synthetic antimalarial drugs and malarial vaccines are currently being developed, but their efficacy against malaria awaits rigorous clinical testing. Artemisinin, a sesquiterpene lactone endoperoxide extracted from Artemisia annua L (family Asteraceae; commonly known as sweet wormwood), is highly effective against multi-drug-resistant Plasmodium spp., but is in short supply and unaffordable to most malaria sufferers. Although total synthesis of artemisinin is difficult and costly, the semi-synthesis of artemisinin or any derivative from microbially sourced Artemisinic acid, its immediate precursor, could be a cost-effective, environmentally friendly, high-quality and reliable source of artemisinin. Here we report the engineering of Saccharomyces cerevisiae to produce high titres (up to 100 mg l(-1)) of Artemisinic acid using an engineered mevalonate pathway, amorphadiene synthase, and a novel cytochrome P450 monooxygenase (CYP71AV1) from A. annua that performs a three-step oxidation of amorpha-4,11-diene to Artemisinic acid. The synthesized Artemisinic acid is transported out and retained on the outside of the engineered yeast, meaning that a simple and inexpensive purification process can be used to obtain the desired product. Although the engineered yeast is already capable of producing Artemisinic acid at a significantly higher specific productivity than A. annua, yield optimization and industrial scale-up will be required to raise Artemisinic acid production to a level high enough to reduce artemisinin combination therapies to significantly below their current prices.
Artemisinic acid inhibits melanogenesis through downregulation of C/EBP alpha-dependent expression of HMG-CoA reductase gene.[Pubmed:23063590]
Food Chem Toxicol. 2013 Jan;51:225-30.
Cholesterol is associated with the regulation of melanogenesis which is the major physiological defense against solar irradiation. The present study was designed to determine the effects of Artemisinic acid on melanogenesis and its mechanisms of action in human epidermal melanocytes. In this study, we found that Artemisinic acid inhibited melanin content. The mRNA levels of microphthalmia-associated transcription factor (MITF) and its downstream genes tyrosinase, tyrosinase-related protein (TRP)-1, and TRP-2 were reduced by Artemisinic acid treatment. Additionally, the mRNA levels of melanogenesis-related genes (c-KIT, stem cell factor (SCF), and macrophage migration inhibitory factor (MIF)) were down-regulated by Artemisinic acid. Furthermore, cAMP production and protein kinase A (PKA) activity were suppressed by Artemisinic acid. Moreover, attempts to elucidate a possible mechanism underlying the Artemisinic acid-mediated effects revealed that Artemisinic acid regulated melanogenesis by inhibiting cholesterol synthesis through downregulation of the hydroxymethylglutaryl CoA (HMG CoA) reductase gene, which was mediated through reduced expression of the CCAAT/enhancer-binding protein (C/EBP) alpha gene. Taken together, these findings indicate that the inhibition of melanogenesis by Artemisinic acid occurs through reduced expression of the HMG CoA reductase gene, which is mediated by C/EBP alpha inhibition and suggest that Artemisinic acid may be useful as a hyperpigmentation inhibitor.
Artemisinic acid is a regulator of adipocyte differentiation and C/EBP delta expression.[Pubmed:22396222]
J Cell Biochem. 2012 Jul;113(7):2488-99.
Adipocyte dysfunction is associated with the development of obesity. In this study, Artemisinic acid, which was isolated from Artemisia annua L., inhibited adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells (hAMSCs) and its mechanism of action was determined. The mRNA levels of peroxidase proliferation-activated receptor (PPAR) gamma and CCAAT/enhancer binding protein (C/EBP) alpha, late adipogenic factors, were reduced by Artemisinic acid. Moreover, the mRNA levels of the PPAR gamma target genes lipoprotein lipase, CD36, adipocyte protein, and liver X receptor were down-regulated by Artemisinic acid. Artemisinic acid reduced expression of the C/EBP delta gene without impacting C/EBP beta. In addition, attempts to elucidate a possible mechanism underlying the Artemisinic acid-mediated effects revealed that reduced expression of the C/EBP delta gene was mediated by inhibiting Jun N-terminal kinase (JNK). Additionally, Artemisinic acid also reduced the expression of the adipogenesis-associated genes glucose transporter-4 and vascular endothelial growth factor. In addition to the interference of Artemisinic acid with adipogenesis, Artemisinic acid significantly attenuated tumor necrosis factor-alpha-induced secretion of interleukin-6 by undifferentiated hAMSCs, thus influencing insulin resistance and the inflammatory state characterizing obesity. Taken together, these findings indicate that inhibiting adipogenic differentiation of hAMSCs by Artemisinic acid occurs primarily through reduced expression of C/EBP delta, which is mediated by the inhibition of JNK and suggest that aremisinic acid may be used as a complementary treatment option for obesity associated with metabolic syndrome.


