Gomisin ACAS# 58546-54-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 58546-54-6 | SDF | Download SDF |
| PubChem ID | 68781 | Appearance | White powder |
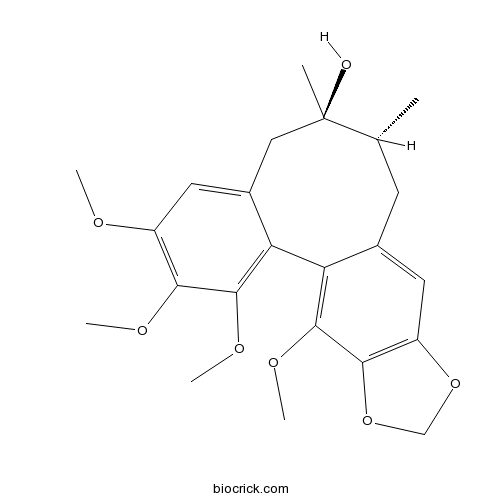
| Formula | C23H28O7 | M.Wt | 416.5 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Synonyms | Gomisin-A; Besigomsin; schizandrol-B; TJN-101; Wuweizi alcohol-B; Wuweizichun-B | ||
| Solubility | DMSO : 50 mg/mL (120.06 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| SMILES | CC1CC2=CC3=C(C(=C2C4=C(C(=C(C=C4CC1(C)O)OC)OC)OC)OC)OCO3 | ||
| Standard InChIKey | ZWRRJEICIPUPHZ-SFDCACGMSA-N | ||
| Standard InChI | InChI=1S/C23H28O7/c1-12-7-13-8-16-20(30-11-29-16)22(28-6)17(13)18-14(10-23(12,2)24)9-15(25-3)19(26-4)21(18)27-5/h8-9,12,24H,7,10-11H2,1-6H3/t12-,23-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gomisin A has anti-inflammatory, antihypertensive, neuroprotective, and anti-proliferation properties, it induces marked protective effects against hepatic and renal injury induced by CCl(4) exposure through differential regulation of the MAPK signal transduction pathway. Gomisin A inhibits COX-2, iNOS, IL-6, TNF-α and NO through the down-regulation of RIP2 and NF-κB activation. |
| Targets | NO | PGE | NOS | TNF-α | IL Receptor | ROS | NADPH-oxidase | TLR | NF-kB | Caspase | Bcl-2/Bax | IkB | COX | STAT | P-gp | ATPase | IKK |
| In vitro | Protective effects of gomisin A isolated from Schisandra chinensis against CCl(4)-induced hepatic and renal injury.[Pubmed: 23381504]Int J Mol Med. 2013 Apr;31(4):888-98.The aim of the present study was to investigate the protective effects of Gomisin A, a lignan compound isolated from Schisandra chinensis, against liver and kidney damage induced by CCl(4) exposure.
Gomisin A enhances tumor necrosis factor-α-induced G1 cell cycle arrest via signal transducer and activator of transcription 1-mediated phosphorylation of retinoblastoma protein.[Pubmed: 23123471]Biol Pharm Bull. 2012;35(11):1997-2003.Gomisin A, a dibenzocyclooctadiene lignan isolated from the fruit of Schisandra chinensis, has been reported as an anti-cancer substance. In this study, we investigated the effects of Gomisin A on cancer cell proliferation and cell cycle arrest in HeLa cells.
|
| In vivo | The molecular mechanisms of the hepatoprotective effect of gomisin A against oxidative stress and inflammatory response in rats with carbon tetrachloride-induced acute liver injury.[Pubmed: 22293346]Biol Pharm Bull. 2012;35(2):171-7.Oxidative damage and inflammation are implicated in the pathogenesis of liver injury and fibrosis. In the present study, we investigated the molecular mechanism by which Gomisin A conferred a hepatoprotective effect, focusing on its antioxidant and anti-inflammatory effects using rats with carbon tetrachloride (CCl(4))-induced acute liver injury.
|
| Kinase Assay | Gomisin A alters substrate interaction and reverses P-glycoprotein-mediated multidrug resistance in HepG2-DR cells.[Pubmed: 16889754 ]Gomisin A inhibits lipopolysaccharide-induced inflammatory responses in N9 microglia via blocking the NF-κB/MAPKs pathway.[Pubmed: 24211520]Food Chem Toxicol. 2014 Jan;63:119-27.Gomisin A, one of the major dibenzocyclooctadiene lignans isolated from Schisandra chinensis Baill., has proved to possess a variety of pharmacological effects. The aim of the present study was to investigate the anti-inflammatory and neuroprotective effects of Gomisin A as well as its potential molecular mechanisms.
Biochem Pharmacol. 2006 Sep 28;72(7):824-37.Through an extensive herbal drug screening program, we found that Gomisin A, a dibenzocyclooctadiene compound isolated from Schisandra chinensis, reversed multidrug resistance (MDR) in Pgp-overexpressing HepG2-DR cells.
|
| Cell Research | Gomisin A decreases the LPS-induced expression of iNOS and COX-2 and activation of RIP2/NF-κB in mouse peritoneal macrophages.[Pubmed: 24749675]Immunopharmacol Immunotoxicol. 2014 Jun;36(3):195-201.Gomisin A (GA), a lignan component contained in the fruit of Schisandra chinensis Baillon, improves hepatic cell degeneration, vasodilatory activity and insulin sensitivity. These effects also impact the immune system, including various inflammatory mediators and cytokines.
In this study, the anti-inflammatory effect of GA on lipopolysaccharide-stimulated mouse peritoneal macrophages was studied.
|
| Animal Research | Antihypertensive effect of gomisin A from Schisandra chinensis on angiotensin II-induced hypertension via preservation of nitric oxide bioavailability.[Pubmed: 22534517 ]Hypertens Res. 2012 Sep;35(9):928-34.Gomisin A (GA) is a small molecular weight lignan present in Schisandra chinensis, and has been demonstrated to have vasodilatory activity.
In the present study, we investigated the effect of GA on blood pressure (BP) in angiotensin II (Ang II)-induced hypertensive mice.
|

Gomisin A Dilution Calculator

Gomisin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.401 mL | 12.0048 mL | 24.0096 mL | 48.0192 mL | 60.024 mL |
| 5 mM | 0.4802 mL | 2.401 mL | 4.8019 mL | 9.6038 mL | 12.0048 mL |
| 10 mM | 0.2401 mL | 1.2005 mL | 2.401 mL | 4.8019 mL | 6.0024 mL |
| 50 mM | 0.048 mL | 0.2401 mL | 0.4802 mL | 0.9604 mL | 1.2005 mL |
| 100 mM | 0.024 mL | 0.12 mL | 0.2401 mL | 0.4802 mL | 0.6002 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Schisandrol B is one of its major active constituents of traditional hepato-protective Chinese medicine, Schisandra sphenanthera. IC50 value: Target: in vitro: SolB pretreatment significantly attenuated the increases in alanine aminotransferase and aspartate aminotransferase activity, and prevented elevated hepatic malondialdehyde formation and the depletion of mitochondrial glutathione (GSH) in a dose-dependent manner. SolB also dramatically altered APAP metabolic activation by inhibiting the activities of CYP2E1 and CYP3A11, which was evidenced by significant inhibition of the formation of the oxidized APAP metabolite NAPQI-GSH [1]. SolB abrogated APAP-induced activation of p53 and p21, and increased expression of liver regeneration and antiapoptotic-related proteins such as cyclin D1 (CCND1), PCNA, and BCL-2.
References:
[1]. Jiang Y, et al. Schisandrol B protects against acetaminophen-induced hepatotoxicity by inhibition of CYP-mediated bioactivation and regulation of liver regeneration. Toxicol Sci. 2015 Jan;143(1):107-15.
[2]. Jin J, et al. Enhancement of oral bioavailability of paclitaxel after oral administration of Schisandrol B in rats. Biopharm Drug Dispos. 2010 May;31(4):264-8.
- Cucurbitacin IIA
Catalog No.:BCN5019
CAS No.:58546-34-2
- Rebaudioside B
Catalog No.:BCN2612
CAS No.:58543-17-2
- Rebaudioside A
Catalog No.:BCN5900
CAS No.:58543-16-1
- (-)-Cephaeline dihydrochloride
Catalog No.:BCN8323
CAS No.:5853-29-2
- Pseurotin A
Catalog No.:BCN7246
CAS No.:58523-30-1
- IOX 1
Catalog No.:BCC6192
CAS No.:5852-78-8
- ent-16beta,17-Isopropylidenedioxykaurane
Catalog No.:BCN1408
CAS No.:58493-71-3
- Olvanil
Catalog No.:BCC6855
CAS No.:58493-49-5
- Lemannine
Catalog No.:BCN3742
CAS No.:58480-54-9
- Platycodin D
Catalog No.:BCN4982
CAS No.:58479-68-8
- Ferrugine
Catalog No.:BCN1910
CAS No.:58471-11-7
- Darlingine
Catalog No.:BCN1906
CAS No.:58471-10-6
- Schisantherin B
Catalog No.:BCN1023
CAS No.:58546-55-7
- Schisantherin A
Catalog No.:BCN1024
CAS No.:58546-56-8
- Confluentin
Catalog No.:BCN5795
CAS No.:585534-03-8
- Losmapimod
Catalog No.:BCC5368
CAS No.:585543-15-3
- Saikosaponin B1
Catalog No.:BCN5917
CAS No.:58558-08-0
- Saikosaponin B4
Catalog No.:BCN8516
CAS No.:58558-09-1
- Anagrelide HCl
Catalog No.:BCC2306
CAS No.:58579-51-4
- m-Anisic acid
Catalog No.:BCC9015
CAS No.:586-38-9
- H- ß-HoGlu-OH.HCl
Catalog No.:BCC3232
CAS No.:58610-41-6
- Boc-ON
Catalog No.:BCC2797
CAS No.:58632-95-4
- PH-797804
Catalog No.:BCC3672
CAS No.:586379-66-0
- Boc-Cys(Acm)-ONp
Catalog No.:BCC3375
CAS No.:58651-76-6
Gomisin A inhibits lipopolysaccharide-induced inflammatory responses in N9 microglia via blocking the NF-kappaB/MAPKs pathway.[Pubmed:24211520]
Food Chem Toxicol. 2014 Jan;63:119-27.
Gomisin A, one of the major dibenzocyclooctadiene lignans isolated from Schisandra chinensis Baill., has proved to possess a variety of pharmacological effects. The aim of the present study was to investigate the anti-inflammatory and neuroprotective effects of Gomisin A as well as its potential molecular mechanisms. It was found that Gomisin A not only inhibited the production of NO and PGE2 in a concentration-dependent manner but also suppressed the expressions of iNOS and COX-2 in LPS-stimulated N9 microglia without observable cytotoxicity. Gomisin A was also able to attenuate the mRNA expression and the production of pro-inflammatory factors TNF-alpha, IL-1beta and IL-6. Moreover, LPS induced reactive oxygen species (ROS) production, NADPH oxidase activation, and gp91phox expression, which were markedly inhibited by Gomisin A in microglia. Furthermore, the data showed that Gomisin A significantly down-regulated the TLR4 protein expression, and inhibited nuclear transcription factor (NF)-kappaB and mitogen-activated protein kinases (MAPKs) signaling pathways. Additionally, Gomisin A alleviated the cell death of SH-SY5Y neuroblastoma, rat primary cortical and hippocampal neurons induced by the conditioned-media from activated microglia. In summary, Gomisin A may exert neuroprotective effects by attenuating the microglia-mediated neuroinflammatory response via inhibiting the TLR4-mediated NF-kappaB and MAPKs signaling pathways.
Antihypertensive effect of gomisin A from Schisandra chinensis on angiotensin II-induced hypertension via preservation of nitric oxide bioavailability.[Pubmed:22534517]
Hypertens Res. 2012 Sep;35(9):928-34.
Gomisin A (GA) is a small molecular weight lignan present in Schisandra chinensis, and has been demonstrated to have vasodilatory activity. In the present study, we investigated the effect of GA on blood pressure (BP) in angiotensin II (Ang II)-induced hypertensive mice. C57/BL6 mice infused subcutaneously with Ang II (1 and 2 mug kg(-)(1) per min for 2 weeks) showed an increase in BP with a decrease in nitric oxide (NO) metabolites in plasma, and a negative correlation between these two parameters was demonstrated. In the thoracic aorta from Ang II-induced hypertensive mice, a decrease in vascular NO that was accompanied by a diminution of phosphorylated endothelial nitric oxide synthase (eNOS), as well as by increased reactive oxygen species (ROS) production, was demonstrated. These alterations in BP, eNOS phosphorylation and ROS production in the vasculature of Ang II-treated mice were markedly and dose-dependently reversed by simultaneous administration of GA (2 and 10 mug kg(-)(1) per min). In addition, Ang II-induced ROS production in cultured vascular cells such as endothelial cells and vascular smooth muscle cells was markedly attenuated by GA. These results suggested that GA attenuated the increase in BP via preservation of vascular NO bioavailability not only by inhibiting ROS production but also by preventing the impairment of eNOS function in the vasculature of Ang II-induced hypertensive mice.
Gomisin A alters substrate interaction and reverses P-glycoprotein-mediated multidrug resistance in HepG2-DR cells.[Pubmed:16889754]
Biochem Pharmacol. 2006 Sep 28;72(7):824-37.
Through an extensive herbal drug screening program, we found that Gomisin A, a dibenzocyclooctadiene compound isolated from Schisandra chinensis, reversed multidrug resistance (MDR) in Pgp-overexpressing HepG2-DR cells. Gomisin A was relatively non-toxic but without altering Pgp expression, it restored the cytotoxic actions of anticancer drugs such as vinblastine and doxorubicin that are Pgp substrates but may act by different mechanisms. Several lines of evidence suggest that Gomisin A alters Pgp-substrate interaction but itself is neither a Pgp substrate nor competitive inhibitor. (1) First unlike Pgp substrates Gomisin A inhibited the basal Pgp-associated ATPase (Pgp-ATPase) activity. (2) The cytotoxicity of Gomisin A was not affected by Pgp competitive inhibitors such as verapamil. (3) Gomisin A acted as an uncompetitive inhibitor for Pgp-ATPase activity stimulated by the transport substrates verapamil and progesterone. (4) On the inhibition of rhodamine-123 efflux the effects of Gomisin A and the competitive inhibitor verapamil were additive, so were the effects of Gomisin A and the ATPase inhibitor vanadate. (5) Binding of transport substrates with Pgp would result in a Pgp conformational change favoring UIC-2 antibody reactivity but Gomisin A impeded UIC-2 binding. (6) Photocrosslinking of Pgp with its transport substrate [125I]iodoarylazidoprazosin was inhibited by Gomisin A in a concentration-dependent manner. Taken together our results suggest that Gomisin A may bind to Pgp simultaneously with substrates and alters Pgp-substrate interaction.
Protective effects of gomisin A isolated from Schisandra chinensis against CCl(4)-induced hepatic and renal injury.[Pubmed:23381504]
Int J Mol Med. 2013 Apr;31(4):888-98.
The aim of the present study was to investigate the protective effects of Gomisin A, a lignan compound isolated from Schisandra chinensis, against liver and kidney damage induced by CCl(4) exposure. We assessed alterations in organ weights, levels of serum biochemical indicators, and activation of the caspase-3 and MAPK signaling pathways and carried out histological analysis of liver and kidney tissue in rats pretreated with Gomisin A for four days. In the Gomisin A/CCl(4)-treated group, only the liver experienced a significant increase in weight, whereas the other organs did not undergo any changes. Five biochemical indicators in serum indicated that liver and kidney toxicity dramatically decreased upon Gomisin A pretreatment, although the decrease in ratios varied. Upon histological analysis, the Gomisin A/CCl(4)-treated group showed less hepatocellular necrosis, a poorly dilated central vein in the liver section, decreased diameter of the glomerulus, a lower number of capillaries, and a convoluted tubule in the kidney section. Furthermore, the formation of active caspase-3 was inhibited by Gomisin A pretreatment in the Gomisin A/CCl(4)-treated group, whereas the expression level of Bax protein was slightly increased. Western blot analysis revealed that there were differences between the liver and kidney in terms of activation of the MAPK signaling pathway. In the liver, Gomisin A pretreatment increased phosphorylation of three members of the MAPK pathway when compared to that in the vehicle pretreatment group. However, in the kidney, only the phosphorylation level of p38 was elevated upon Gomisin A pretreatment, whereas levels of the other two members were decreased. These results suggest that Gomisin A induces marked protective effects against hepatic and renal injury induced by CCl(4) exposure through differential regulation of the MAPK signal transduction pathway.
The molecular mechanisms of the hepatoprotective effect of gomisin A against oxidative stress and inflammatory response in rats with carbon tetrachloride-induced acute liver injury.[Pubmed:22293346]
Biol Pharm Bull. 2012;35(2):171-7.
Oxidative damage and inflammation are implicated in the pathogenesis of liver injury and fibrosis. In the present study, we investigated the molecular mechanism by which Gomisin A conferred a hepatoprotective effect, focusing on its antioxidant and anti-inflammatory effects using rats with carbon tetrachloride (CCl(4))-induced acute liver injury. Pretreatment with Gomisin A prior to the administration of CCl(4) markedly prevented an increase in alanine aminotransferase, aspartate aminotransferase, and histological hepatic lesions. Gomisin A was also associated with a decrease in hepatic lipid peroxidation, and increased superoxide dismutase activity, suggesting that Gomisin A has an antioxidant effect. In addition Gomisin A treatment ameliorated mRNA levels of CCl(4)-induced inflammatory mediators, including tumor necrosis factor-alpha, interleukin-1beta and inducible nitric oxide (NO) synthase, and the protein levels of transcriptional upregulator nuclear factor kappa B (NF-kappaB) and phospho-inhibitor of NF-kappaB (IkappaB). Furthermore, alpha-smooth muscle actin (alpha-SMA), a myofibroblast marker, was also inhibited by Gomisin A treatment. These results suggest that Gomisin A inhibits the oxidative stress and activation of NF-kappaB, leading to down-regulation of pro-inflammatory mediators and amelioration of fibrogenesis.
Gomisin A enhances tumor necrosis factor-alpha-induced G1 cell cycle arrest via signal transducer and activator of transcription 1-mediated phosphorylation of retinoblastoma protein.[Pubmed:23123471]
Biol Pharm Bull. 2012;35(11):1997-2003.
Gomisin A, a dibenzocyclooctadiene lignan isolated from the fruit of Schisandra chinensis, has been reported as an anti-cancer substance. In this study, we investigated the effects of Gomisin A on cancer cell proliferation and cell cycle arrest in HeLa cells. Gomisin A significantly inhibited cell proliferation in a dose-dependent manner after 72 h treatment, especially in the presence of tumor necrosis factor-alpha (TNF-alpha), due to cell cycle arrest in the G1 phase with the downregulation of cyclin D1 expression and Retinoblastoma (RB) phosphorylation. In addition, Gomisin A in combination with TNF-alpha strongly suppressed the expression of signal transducer and activator of transcription 1 (STAT1). Inhibition of STAT1 pathways by a small-interfering RNA against STAT1 and AG490 Janus kinase (JAK) kinase inhibitor AG490 reduced the cyclin D1 expression and RB phosphorylation, indicating that JAK-mediated STAT1 activation is involved in Gomisin A-induced G1 cell cycle arrest.
Gomisin A decreases the LPS-induced expression of iNOS and COX-2 and activation of RIP2/NF-kappaB in mouse peritoneal macrophages.[Pubmed:24749675]
Immunopharmacol Immunotoxicol. 2014 Jun;36(3):195-201.
Gomisin A (GA), a lignan component contained in the fruit of Schisandra chinensis Baillon, improves hepatic cell degeneration, vasodilatory activity and insulin sensitivity. These effects also impact the immune system, including various inflammatory mediators and cytokines. In this study, the anti-inflammatory effect of GA on lipopolysaccharide-stimulated mouse peritoneal macrophages was studied. Pretreatment with GA attenuated the expression of receptor-interacting protein 2 (RIP2) and IkappaB kinase-beta (IKK-beta) as well as IKK-beta phosphorylation. The activation of nuclear factor-kappa B (NF-kappaB) in the nucleus, the phosphorylation of IkappaBalpha and degradation of IkappaBalpha in the cytosol were suppressed by GA. GA decreased the production and mRNA expression of the inflammatory cytokines tumor necrosis factor-alpha (TNF-alpha) and interleukin (IL)-6. In addition, expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) and production of nitric oxide were decreased by pretreatment with GA. In conclusion, these results show that the anti-inflammatory properties of GA potentially result from the inhibition of COX-2, iNOS, IL-6, TNF-alpha and NO through the down-regulation of RIP2 and NF-kappaB activation. These results impact the development of potential health products for preventing and treating inflammatory diseases.


