FerrugineCAS# 58471-11-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 58471-11-7 | SDF | Download SDF |
| PubChem ID | 134715032 | Appearance | Powder |
| Formula | C15H19NO | M.Wt | 229.32 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
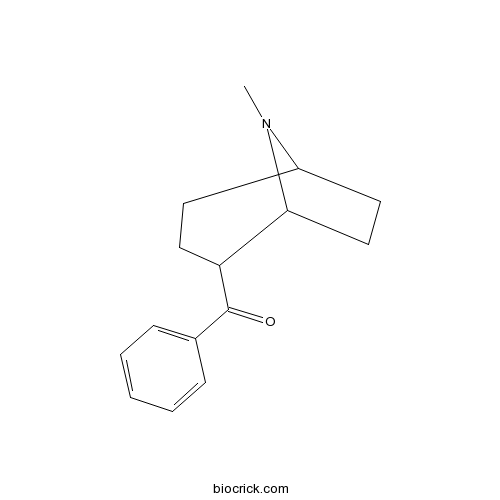
| Chemical Name | [(1S,5S)-8-methyl-8-azabicyclo[3.2.1]octan-2-yl]-phenylmethanone | ||
| SMILES | CN1C2CCC(C1CC2)C(=O)C3=CC=CC=C3 | ||
| Standard InChIKey | FKBXXVPBVMWIDS-HPNRGHHYSA-N | ||
| Standard InChI | InChI=1S/C15H19NO/c1-16-12-7-9-13(14(16)10-8-12)15(17)11-5-3-2-4-6-11/h2-6,12-14H,7-10H2,1H3/t12-,13?,14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Tetrahedron,2012,68(39): 8236–8244.Approaches to the enantioselective synthesis of ferrugine and its analogues[Reference: WebLink]A four-step synthetic route, to Ferrugine (2α-benzoyltropane), its methyl analogue (2-acetyltropane) and their N-benzyl analogues is reported.
Tetrahedron Letters,2009,50(51):7196–7198.Enantioselective route to ferrugine and its methyl analogue via aldol deoxygenation[Reference: WebLink]A simple enantioselective approach to Ferrugine (2α-benzoyltropane) and its methyl analogue (2-acetyltropane) is reported.
|

Ferrugine Dilution Calculator

Ferrugine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3607 mL | 21.8036 mL | 43.6072 mL | 87.2144 mL | 109.018 mL |
| 5 mM | 0.8721 mL | 4.3607 mL | 8.7214 mL | 17.4429 mL | 21.8036 mL |
| 10 mM | 0.4361 mL | 2.1804 mL | 4.3607 mL | 8.7214 mL | 10.9018 mL |
| 50 mM | 0.0872 mL | 0.4361 mL | 0.8721 mL | 1.7443 mL | 2.1804 mL |
| 100 mM | 0.0436 mL | 0.218 mL | 0.4361 mL | 0.8721 mL | 1.0902 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Darlingine
Catalog No.:BCN1906
CAS No.:58471-10-6
- N-(2-Hydroxy-4-methoxyphenyl)acetamide
Catalog No.:BCN1409
CAS No.:58469-06-0
- H-D-Leu-OMe.HCl
Catalog No.:BCC2681
CAS No.:5845-53-4
- Angiotensin 1/2 (1-5)
Catalog No.:BCC1035
CAS No.:58442-64-1
- Boc-2-Nal-OH
Catalog No.:BCC3289
CAS No.:58438-04-3
- H-2-Nal-OH.HCl
Catalog No.:BCC3287
CAS No.:58438-03-2
- Dihydroresveratrol
Catalog No.:BCN5793
CAS No.:58436-28-5
- DL-Demethylcoclaurine
Catalog No.:BCC8317
CAS No.:5843-65-2
- Isopimaric acid
Catalog No.:BCN4618
CAS No.:5835-26-7
- 4,4'-Bis(N-carbazolyl)-1,1'-biphenyl
Catalog No.:BCC8660
CAS No.:58328-31-7
- Saikosaponin B3
Catalog No.:BCN8178
CAS No.:58316-42-0
- Saikosaponin B2
Catalog No.:BCN5916
CAS No.:58316-41-9
- Platycodin D
Catalog No.:BCN4982
CAS No.:58479-68-8
- Lemannine
Catalog No.:BCN3742
CAS No.:58480-54-9
- Olvanil
Catalog No.:BCC6855
CAS No.:58493-49-5
- ent-16beta,17-Isopropylidenedioxykaurane
Catalog No.:BCN1408
CAS No.:58493-71-3
- IOX 1
Catalog No.:BCC6192
CAS No.:5852-78-8
- Pseurotin A
Catalog No.:BCN7246
CAS No.:58523-30-1
- (-)-Cephaeline dihydrochloride
Catalog No.:BCN8323
CAS No.:5853-29-2
- Rebaudioside A
Catalog No.:BCN5900
CAS No.:58543-16-1
- Rebaudioside B
Catalog No.:BCN2612
CAS No.:58543-17-2
- Cucurbitacin IIA
Catalog No.:BCN5019
CAS No.:58546-34-2
- Gomisin A
Catalog No.:BCN5794
CAS No.:58546-54-6
- Schisantherin B
Catalog No.:BCN1023
CAS No.:58546-55-7
Thermal elimination of diethyldithiocarbamates and application in the synthesis of (+/-)-ferrugine.[Pubmed:18785775]
J Org Chem. 2008 Oct 17;73(20):8116-9.
Dithiocarbamate-substituted lactams, prepared through group-transfer cyclization reactions of carbamoyl radicals, undergo a Chugaev-like thermal elimination of the dithiocarbamate group in refluxing diphenyl ether to form alpha,beta- and/or beta,gamma-unsaturated amides, depending on the structure of the starting material. This reaction sequence was used to prepare an unsaturated [3.2.2] bridged bicyclic amide, which was converted in a one-pot procedure to the 8-azabicyclo[3.2.1]octane ring system of the tropane alkaloid Ferrugine by treatment with phenyllithium followed by aqueous sodium hydroxide.


