GYKI 47261 dihydrochlorideAMPA receptor antagonist, non-competitive and selective CAS# 1217049-32-5 |
- Pioglitazone HCl
Catalog No.:BCC2278
CAS No.:112529-15-4
- Rosiglitazone
Catalog No.:BCC2264
CAS No.:122320-73-4
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- T0070907
Catalog No.:BCC2261
CAS No.:313516-66-4
- Troglitazone
Catalog No.:BCC2016
CAS No.:97322-87-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1217049-32-5 | SDF | Download SDF |
| PubChem ID | 46861544 | Appearance | Powder |
| Formula | C18H17Cl3N4 | M.Wt | 395.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | 4-(8-chloro-2-methyl-11H-imidazo[1,2-c][2,3]benzodiazepin-6-yl)aniline;dihydrochloride | ||
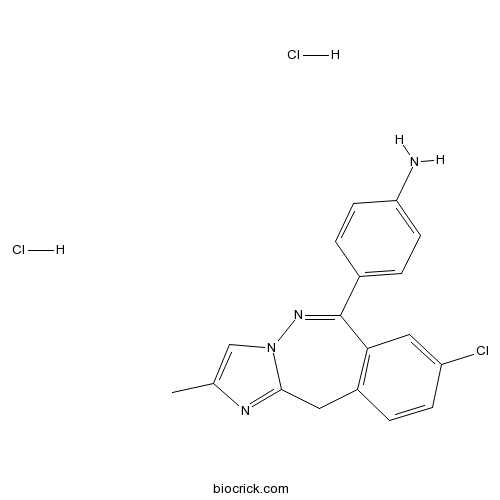
| SMILES | CC1=CN2C(=N1)CC3=C(C=C(C=C3)Cl)C(=N2)C4=CC=C(C=C4)N.Cl.Cl | ||
| Standard InChIKey | HUNSEMNKAFDVDI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H15ClN4.2ClH/c1-11-10-23-17(21-11)8-13-2-5-14(19)9-16(13)18(22-23)12-3-6-15(20)7-4-12;;/h2-7,9-10H,8,20H2,1H3;2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-competitive AMPA receptor antagonist (IC50 = 2.5 μM). Displays broad spectrum anticonvulsive activity and neuroprotective effects in vivo. |

GYKI 47261 dihydrochloride Dilution Calculator

GYKI 47261 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5271 mL | 12.6355 mL | 25.271 mL | 50.5421 mL | 63.1776 mL |
| 5 mM | 0.5054 mL | 2.5271 mL | 5.0542 mL | 10.1084 mL | 12.6355 mL |
| 10 mM | 0.2527 mL | 1.2636 mL | 2.5271 mL | 5.0542 mL | 6.3178 mL |
| 50 mM | 0.0505 mL | 0.2527 mL | 0.5054 mL | 1.0108 mL | 1.2636 mL |
| 100 mM | 0.0253 mL | 0.1264 mL | 0.2527 mL | 0.5054 mL | 0.6318 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GYKI 47261 dihydrochloride is a selective and non-competitive antagonist of AMPA receptor with IC50 value of 2.5 μM [1].
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA receptor) is an ionotropic transmembrane receptor for glutamate and mediates fast synaptic transmission in the central nervous system. AMPA receptors are oligomeric assemblies of four protein subunits, GluR1-4.
GYKI 47261 dihydrochloride is a selective and non-competitive AMPA receptor antagonist. In isolated cerebellar Purkinje cells, GYKI 47261 (10 μM) inhibited currents induced by kainate or AMPA in a non-competitive way [1]. In rat hepatocytes, GYKI-47261 (10 μM) significantly increased CYP2E1 activity. In human hepatocytes, GYKI-47261 produced the maximal induction at 0.01 μM [2].
In mice, GYKI 47261 induced muscle relaxant effects with ED50 values of 15.8-36.5 mg/kg. In a transient focal ischemia rat model, GYKI 47261 significantly reduced infarct size by 62.3-67.4%. In mice, GYKI 47261 effectively reduced oxotremorine-induced tremor and inhibited MPTP-induced neurotoxicity [1]. In Parkinson’s disease rat model, administration of GYKI-47261 and MK-801 completely normalized the response shortening induced by levodopa. In monkeys, administration of amantadine and GYKI-47261 significantly reduced dyskinesias induced by levodopa by 51% [3].
References:
[1]. Abrahám G, Sólyom S, Csuzdi E, et al. New non competitive AMPA antagonists. Bioorg Med Chem, 2000, 8(8): 2127-2143.
[2]. Tamási V, Hazai E, Porsmyr-Palmertz M, et al. GYKI-47261, a new AMPA [2-amino-3-(3-hydroxymethylisoxazole-4-yl)propionic acid] antagonist, is a CYP2E1 inducer. Drug Metab Dispos, 2003, 31(11): 1310-1314.
[3]. Bibbiani F, Oh JD, Kielaite A, et al. Combined blockade of AMPA and NMDA glutamate receptors reduces levodopa-induced motor complications in animal models of PD. Exp Neurol, 2005, 196(2): 422-429.
- BU 239 hydrochloride
Catalog No.:BCC5668
CAS No.:1217041-98-9
- PTC209 HBr
Catalog No.:BCC5640
CAS No.:1217022-63-3
- Moluccanin diacetate
Catalog No.:BCN6108
CAS No.:121700-27-4
- Moluccanin
Catalog No.:BCN6107
CAS No.:121700-26-3
- Sarafotoxin S6c
Catalog No.:BCC5721
CAS No.:121695-87-2
- CGP 20712 dihydrochloride
Catalog No.:BCC6893
CAS No.:1216905-73-5
- ZK 93426 hydrochloride
Catalog No.:BCC7229
CAS No.:1216792-30-1
- GSK 4112
Catalog No.:BCC7741
CAS No.:1216744-19-2
- BYK 191023 dihydrochloride
Catalog No.:BCC7506
CAS No.:1216722-25-6
- SCH 79797 dihydrochloride
Catalog No.:BCC7125
CAS No.:1216720-69-2
- Trap 101
Catalog No.:BCC7390
CAS No.:1216621-00-9
- 2-Cyclopropyl-4-(4-fluorophenyl)quinoline-3-carboxaldehyde
Catalog No.:BCC8573
CAS No.:121660-37-5
- 6-O-p-Methoxycinnamoylcatalpol
Catalog No.:BCN6109
CAS No.:121710-02-9
- CP 31398 dihydrochloride
Catalog No.:BCC2406
CAS No.:1217195-61-3
- A 350619 hydrochloride
Catalog No.:BCC5939
CAS No.:1217201-17-6
- VD2-D3
Catalog No.:BCC2034
CAS No.:1217448-46-8
- Ro 26-4550 trifluoroacetate
Catalog No.:BCC5813
CAS No.:1217448-66-2
- Isoderrone
Catalog No.:BCN3698
CAS No.:121747-89-5
- Isochandalone
Catalog No.:BCN4767
CAS No.:121747-90-8
- (+)-UH 232 maleate
Catalog No.:BCC6790
CAS No.:1217473-50-1
- NAS-181
Catalog No.:BCC7056
CAS No.:1217474-40-2
- threo-1-C-Syringylglycerol
Catalog No.:BCN6110
CAS No.:121748-11-6
- BYL-719
Catalog No.:BCC3707
CAS No.:1217486-61-7
- SB 205607 dihydrobromide
Catalog No.:BCC5687
CAS No.:1217628-73-3
Combined blockade of AMPA and NMDA glutamate receptors reduces levodopa-induced motor complications in animal models of PD.[Pubmed:16203001]
Exp Neurol. 2005 Dec;196(2):422-9.
AMPA and NMDA receptors, abundantly expressed on striatal medium spiny neurons, have been implicated in the regulation of corticostriatal synaptic efficacy. To evaluate the contribution of both glutamate receptor types to the pathogenesis of motor response alterations associated with dopaminergic treatment, we studied the ability of the selective AMPA receptor antagonist GYKI-47261 and the selective NMDA receptor antagonists, MK-801 and amantadine, to mitigate these syndromes in rodent and primate models of Parkinson's disease. The effects of GYKI-47261 and amantadine (or MK-801), alone and in combination, were compared for their ability to modify dyskinesias induced by levodopa. In rats, simultaneous administration of subthreshold doses of AMPA and NMDA receptor antagonists completely normalized the wearing-off response to acute levodopa challenge produced by chronic levodopa treatment (P < 0.05). In primates, the glutamate antagonists GYKI-47261 and amantadine, co-administered at low doses (failing to alter dyskinesia scores), reduced levodopa-induced dyskinesias by 51% (P < 0.05). The simultaneous AMPA and NMDA receptor blockade acts to provide a substantially greater reduction in the response alterations induced by levodopa than inhibition of either of these receptors alone. The results suggest that mechanisms mediated by both ionotropic glutamate receptors make an independent contribution to the pathogenesis of these motor response changes and further that a combination of both drug types may provide relief from these disabling complications at lower and thus safer and more tolerable doses than required when either drug is used alone.
New non competitive AMPA antagonists.[Pubmed:11003158]
Bioorg Med Chem. 2000 Aug;8(8):2127-43.
New halogen atom substituted 2,3-benzodiazepine derivatives condensed with an azole ring on the seven membered part of the ring system of type 3 and 4 as well as 5 and 6 were synthesized. It was found that chloro-, dichloro- and bromo-substitutions in the benzene ring and additionally imidazole ring condensation on the diazepine ring can successfully substitute the methylenedioxy group in the well known molecules GYKI 52466 (1) and GYKI 53773 (2) and the 3-acetyl-4-methyl structural feature in 2, respectively, preserving the highly active AMPA antagonist characteristic of the original molecules. From the most active compounds (3b,i) 3b (GYKI 47261) was chosen for detailed investigations. 3b revealed an excellent, broad spectrum anticonvulsant activity against seizures evoked by electroshock and different chemoconvulsive agents indicating a possible antiepileptic efficacy. 3b was found to be highly active in a transient model of focal ischemia predictive of a therapeutic value in human stroke. 3b also reversed the dopamine depleting effect of MPTP and antagonized the oxotremorine induced tremor in mice indicating a potential antiparkinson activity.


