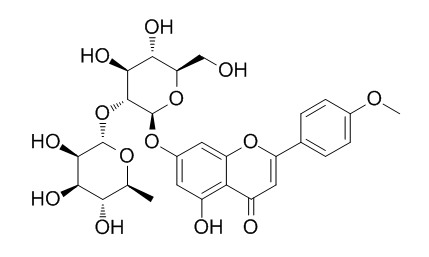
FortunellinCAS# 20633-93-6 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 20633-93-6 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C28H32O14 | M.Wt | 592.6 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fortunellin is a potential anti-inflammation agent in inflammatory diseases, it targets miR-374a, which is a negative regulator of phosphatase and tensin homolog (PTEN). Fortunellin protects against high fructose-induced diabetic heart injury in mice by suppressing inflammation and oxidative stress via AMPK/Nrf-2 pathway regulation. | |||||

Fortunellin Dilution Calculator

Fortunellin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6875 mL | 8.4374 mL | 16.8748 mL | 33.7496 mL | 42.187 mL |
| 5 mM | 0.3375 mL | 1.6875 mL | 3.375 mL | 6.7499 mL | 8.4374 mL |
| 10 mM | 0.1687 mL | 0.8437 mL | 1.6875 mL | 3.375 mL | 4.2187 mL |
| 50 mM | 0.0337 mL | 0.1687 mL | 0.3375 mL | 0.675 mL | 0.8437 mL |
| 100 mM | 0.0169 mL | 0.0844 mL | 0.1687 mL | 0.3375 mL | 0.4219 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Vitexin 2''-glucoside
Catalog No.:BCN0124
CAS No.:61360-94-9
- Acetoxyvalerenic acid
Catalog No.:BCN0123
CAS No.:84638-55-1
- Gomisin U
Catalog No.:BCN0122
CAS No.:135095-46-4
- k-Strophanthoside
Catalog No.:BCN0121
CAS No.:33279-57-1
- (+)-Longifolene
Catalog No.:BCN0120
CAS No.:475-20-7
- VX-702
Catalog No.:BCN0119
CAS No.:479543-46-9
- Lactupicrin
Catalog No.:BCN0118
CAS No.:65725-11-3
- Tricetinidin chloride
Catalog No.:BCN0117
CAS No.:65618-21-5
- (-)-Carveol
Catalog No.:BCN0116
CAS No.:99-48-9
- Sempervirine nitrate
Catalog No.:BCN0115
CAS No.:17994-15-9
- (+)-Isocorydine hydrochloride
Catalog No.:BCN0114
CAS No.:13552-72-2
- alpha-Pinene oxide
Catalog No.:BCN0113
CAS No.:1686-14-2
- 3',5'-Dimethoxy-4'-hydroxyacetophenone
Catalog No.:BCN0126
CAS No.:2478-38-8
- Bacosine
Catalog No.:BCN0127
CAS No.:198014-94-7
- Laudanosine
Catalog No.:BCN0128
CAS No.:1699-51-0
- Negundoside
Catalog No.:BCN0129
CAS No.:82451-20-5
- 2,3-Dehydrosilybin A
Catalog No.:BCN0130
CAS No.:25166-14-7
- (-)-Cadin-4,10(15)-dien-11-oic acid
Catalog No.:BCN0131
CAS No.:1124353-23-6
- (+)-Lariciresinol 4'-O-beta-D-Glucopyranosyl-(1->3)-beta-D-glucopyranoside
Catalog No.:BCN0132
CAS No.:639857-95-7
- 3',4',7,8-Tetramethoxyflavone
Catalog No.:BCN0133
CAS No.:65548-55-2
- Castalin
Catalog No.:BCN0134
CAS No.:19086-75-0
- Protocetraric acid
Catalog No.:BCN0135
CAS No.:489-51-0
- 5,7-Dihydroxy-3',4',5'-trimethoxyflavanone
Catalog No.:BCN0136
CAS No.:62252-10-2
- Bidenoside C
Catalog No.:BCN0137
CAS No.:700877-55-0
Fortunellin-Induced Modulation of Phosphatase and Tensin Homolog by MicroRNA-374a Decreases Inflammation and Maintains Intestinal Barrier Function in Colitis.[Pubmed:29472916]
Front Immunol. 2018 Jan 26;9:83.
Activation of phosphatase and tensin homolog (PTEN) is known to induce cell apoptosis. MicroRNA-374a (miR-374a), which can suppress PTEN expression, has been found abnormally expressed in inflammatory bowel disease (IBD). Fortunellin is a citrus flavonoid that is a potential anti-inflammation agent in inflammatory diseases. The present study investigated the effects and mechanisms underlying Fortunellin-induced inhibition of PTEN in IBD. Colitis was established in rats by the intracolonic administration of 2,4,6-trinitrobenzene sulfonic acid to mimic human ulcerative colitis, which is the main type of IBD. miR-374a expression was measured by quantitative real-time polymerase chain reaction, and the regulation of PTEN by miR-374a was evaluated by dual luciferase reporter assay. Western blotting was used to measure the corresponding protein expression. Fortunellin ameliorated colitis symptoms, including excessive inflammation and oxidative stress. Fortunellin decreased epithelial cell apoptosis through inhibiting PTEN expression in colitis. Fortunellin-induced downregulation of PTEN could be counteracted by miR-374a depletion. Moreover, knockdown of miR-374a in vivo partly inhibited the effects of Fortunellin on rat colitis. In conclusion, PTEN inhibition contributes to the amelioration effects of Fortunellin on colitis. It was confirmed that Fortunellin targets miR-374a, which is a negative regulator of PTEN. This study provides novel insights into the pathological mechanisms and treatment alternatives of colitis.
Fortunellin protects against high fructose-induced diabetic heart injury in mice by suppressing inflammation and oxidative stress via AMPK/Nrf-2 pathway regulation.[Pubmed:28624452]
Biochem Biophys Res Commun. 2017 Aug 19;490(2):552-559.
Inflammation and oxidative stress contribute to the progression of diabetic cardiomyopathy (DCM). The study was first designed to calculate the role of an anti-inflammatory and anti-oxidant Fortunellin (For) in high fructose-induced cardiac injury in diabetic mice. Fortunellin was found to be none of toxicity to mice and cells using various assays. High fructose was used to induce mice with diabetes. The heart histopathological changes and cardiac function were measured. Fortunellin significantly attenuated the score of histopathological alterations and alleviated heart function, accompanied with reduced inflammation and oxidative stress. The pro-inflammatory cytokines and the expression of p-IkappaB kinase alpha (IKKalpha), p-IkappaBalpha, and p-nuclear factor-kappaB (NF-kappaB) were dramatically reduced by Fortunellin, while superoxide dismutase (SOD), catalase (CAT), heme oxygenase-1 (HO-1) and p-AMP-activated protein kinase (AMPK) were significantly enhanced. Moreover, in H9C2 cells with nuclear factor erythroid 2-related factor 2 (Nrf2) knock-down abolished the prevention of Fortunellin against cardiac injury, proved by elevated inflammatory response and oxidative stress. Suppression of p-AMPK reduced the level of Nrf2 and HO-1 induced by Fortunellin, eliminating the protective role of Fortunellin. For the first time, our study suggested that Fortunellin protected against fructose-induced inflammation and oxidative stress by enhancing AMPK/Nrf2 pathway in diabetic mice and cardiomyocytes with fructose treatment.
Phenolic content, antioxidant activity and effective compounds of kumquat extracted by different solvents.[Pubmed:26616917]
Food Chem. 2016 Apr 15;197(Pt A):1-6.
The total phenolic and flavonoid content of extracts from peel of kumquat were higher than those from pulp, and those extracted from immature kumquat were higher than those from mature kumquat. The highest levels of phenolic and flavonoid content were obtained in hot water extracts. The flavonoids of kumquat extracted from hot water were mainly soluble conjugated compounds, including C-glycosides, such as 3',5'-di-C-beta-glucopyranosylphloretin (DGPP), acacetin 8-C-neohesperidoside (margaritene), acacetin 6-C-neohesperidoside (isomargaritene), apigenin 8-C-neohesperidoside, and O-glycosides, such as acacetin 7-O-neohesperidoside (Fortunellin), isosakuranetin 7-O-neohesperidoside (poncirin) and apigenin 7-O-neohesperidoside (rhoifolin). A positive relationship existed between total phenolic content and DPPH scavenging potency (p<0.001). Total flavonoid content showed a similar correlation (p<0.001) to DPPH scavenging potency. The effective flavonoids contributing to antioxidant activity were DGPP and apigenin 8-C-neohesperidoside, which could be extracted in high amounts, by hot water at 90 degrees C, from immature kumquat peel.
Drying effect on flavonoid composition and antioxidant activity of immature kumquat.[Pubmed:25308680]
Food Chem. 2015 Mar 15;171:356-63.
A seven flavonoids in hot water extract of immature kumquat (Citrus japonica var. margarita) were identified and quantified (mg/100g fresh fruit): 3',5'-di-C-beta-glucopyranosylphloretin (DGPP, 285.9 +/- 2.9 mg/100g), acacetin 8-C-neohesperidoside (margaritene, 136.2 +/- 2.6 mg/100g), acacetin 6-C-neohesperidoside (isomargaritene, 119.1 +/- 1.8 mg/100g), Fortunellin (acacetin 7-O-neohesperidoside, 28.5 +/- 0.7 mg/100g), apigenin 8-C-neohesperidoside (16.9 +/- 0.1mg/100g), poncirin (isosakuranetin 7-O-neohesperidoside, 5.1 +/- 0.1mg/100g), and rhoifolin (apigenin 7-O-neohesperidoside, 2.0 +/- 0.1mg/100g). When immature kumquat was dried at 110 and 130 degrees C for 0.5h, the antioxidant activity, total phenolic content and identified flavonoids increased. The UV absorbance of browning products of immature kumquat dried at 130 degrees C for 1.5h increased dramatically, while the identified flavonoids decreased. Therefore, it was concluded that drying below 130 degrees C for 1.0 h, could release phenolic compounds, which resulted in the increasing antioxidant activity. Drying at 130 degrees C for 1.5h, it might be due to the effect of formed browning products.
[Chemical constituents of Fortunella margarita fruits].[Pubmed:25174109]
Zhong Yao Cai. 2014 Mar;37(3):435-8.
OBJECTIVE: To study the chemical constituents of the fruits of Fortunella margarita. METHODS: The constituents were isolated and purified on silica gel column and other column chromatography, and their structures were determined by means of spectral techniques and physicochemical data. RESULTS: 11 compounds were isolated and identified as Fortunellin (1), naringenin (2), phloridzin (3), nicotinflorin (4), rhoifolin (5), 4'-methoxy vitexin-2"-O-alpha-L-rhamnopyranoside (6), 4'-methoxy isovitexin-2"-O-alpha-L-rhamnopyranoside (7), rutin (8), phloretin-3', 5'-di-C-beta-glucopyranoside (9), 5-hydroxymethyl-furaldehyde (10) and beta-sitosterol (11). CONCLUSION: Compound 2 - 4,7 and 10 are isolated from the plant for the first time.


