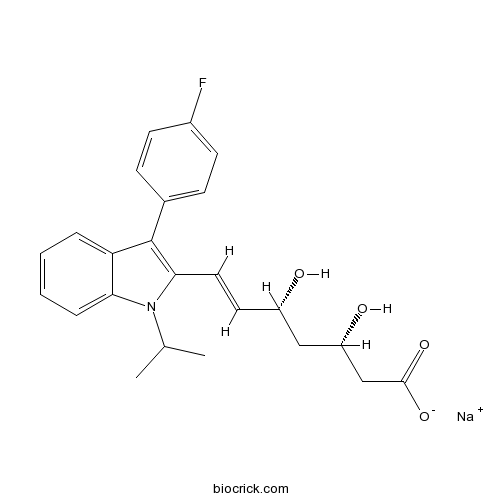
Fluvastatin SodiumHMG-CoA reductase inhibitor CAS# 93957-55-2 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 93957-55-2 | SDF | Download SDF |
| PubChem ID | 23679527 | Appearance | Powder |
| Formula | C24H25FNNaO4 | M.Wt | 433.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 50 mg/mL (115.35 mM; Need ultrasonic) | ||
| Chemical Name | sodium;(E,3S,5R)-7-[3-(4-fluorophenyl)-1-propan-2-ylindol-2-yl]-3,5-dihydroxyhept-6-enoate | ||
| SMILES | CC(C)N1C2=CC=CC=C2C(=C1C=CC(CC(CC(=O)[O-])O)O)C3=CC=C(C=C3)F.[Na+] | ||
| Standard InChIKey | ZGGHKIMDNBDHJB-RPQBTBOMSA-M | ||
| Standard InChI | InChI=1S/C24H26FNO4.Na/c1-15(2)26-21-6-4-3-5-20(21)24(16-7-9-17(25)10-8-16)22(26)12-11-18(27)13-19(28)14-23(29)30;/h3-12,15,18-19,27-28H,13-14H2,1-2H3,(H,29,30);/q;+1/p-1/b12-11+;/t18-,19-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Orally active, potent and competitive HMG-CoA reductase inhibitor (IC50 = 40 -100 nM at human liver microsomes). Inhibits vascular smooth muscle proliferation in vitro (IC50 = 70 nM) and exhibits antihypercholesterolemic and antioxidant activity in vivo. |

Fluvastatin Sodium Dilution Calculator

Fluvastatin Sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3071 mL | 11.5354 mL | 23.0707 mL | 46.1414 mL | 57.6768 mL |
| 5 mM | 0.4614 mL | 2.3071 mL | 4.6141 mL | 9.2283 mL | 11.5354 mL |
| 10 mM | 0.2307 mL | 1.1535 mL | 2.3071 mL | 4.6141 mL | 5.7677 mL |
| 50 mM | 0.0461 mL | 0.2307 mL | 0.4614 mL | 0.9228 mL | 1.1535 mL |
| 100 mM | 0.0231 mL | 0.1154 mL | 0.2307 mL | 0.4614 mL | 0.5768 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fluvastatin sodium is an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase [1].
Fluvastatin is a drug used to treat patients with hypercholesterolaemia. It lowers serum total cholesterol (TC) and low density lipoprotein cholesterol (LDL-C) as well as the serum apolipoprotein B. It also increases high density lipoprotein cholesterol (HDL-C) levels and apolipoprotein A-I levels. In addition to these, fluvastatin has antiatherogenic, antithrombotic and antioxidant effects. In U937 human monocyte cells, fluvastatin reduces the expression of adhesion molecules thus reducing the interaction between monocytes and endothelial cells. Fluvastatin dose-dependently reduces the platelet aggregation in vitro. The administration of fluvastatin at dose of 40 mg/day to patients reduces platelet aggregation by 10 to 15% after 4 to 24 weeks. In patients with hypercholesterolaemia, fluvastatin 40 mg/day for 8 weeks can reduce copper-induced diene production in LDL-C isolated from these patients. It is also reported that, fluvastatin can inhibit the production of NO and decreases inflammatory angiogenesis induced by the sponge matrix [1, 2].
References:
[1] Langtry HD, Markham A. Fluvastatin. A Review of its Use in Lipid Disorders. Drugs. 1999 Apr;57(4):583-606.
[2] Fernanda A. Araujo, Monaliza A. Rocha, Luciano S. A. Capettini, Paula P. Campos, Monica A. N. D. Ferreira, Virginia S. Lemos1and Silvia P. Andrade. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor (fluvastatin) decreases inflammatory angiogenesis in mice. APMIS. 2012,121:422-430.
- Fluvastatin
Catalog No.:BCC1579
CAS No.:93957-54-1
- Toonaciliatin M
Catalog No.:BCN7881
CAS No.:93930-04-2
- Hirsutanonol 5-O-glucoside
Catalog No.:BCN4485
CAS No.:93915-36-7
- FIPI
Catalog No.:BCC7721
CAS No.:939055-18-2
- 9-Oxo-2,7-bisaboladien-15-oic acid
Catalog No.:BCN4484
CAS No.:93888-59-6
- Isochamaejasmine
Catalog No.:BCN3128
CAS No.:93859-63-3
- KU-0063794
Catalog No.:BCC2484
CAS No.:938440-64-3
- ATPγS tetralithium salt
Catalog No.:BCC7855
CAS No.:93839-89-5
- 22-beta-Acetoxyglycyrrhizin
Catalog No.:BCN7904
CAS No.:938042-17-2
- 3-Prenyl-2,4,6-trihydroxybenzophenone
Catalog No.:BCN1303
CAS No.:93796-20-4
- Roxatidine Acetate HCl
Catalog No.:BCC4534
CAS No.:93793-83-0
- gamma-Secretase Modulators
Catalog No.:BCC1586
CAS No.:937812-80-1
- [Ac-Tyr1,D-Phe2]GRF 1-29, amide (human)
Catalog No.:BCC5719
CAS No.:93965-89-0
- PF-00562271
Catalog No.:BCC3684
CAS No.:939791-38-5
- ACTB-1003
Catalog No.:BCC5587
CAS No.:939805-30-8
- BI 6015
Catalog No.:BCC6249
CAS No.:93987-29-2
- p53 and MDM2 proteins-interaction-inhibitor chiral
Catalog No.:BCC1830
CAS No.:939981-37-0
- RG7112
Catalog No.:BCC1894
CAS No.:939981-39-2
- p53 and MDM2 proteins-interaction-inhibitor racemic
Catalog No.:BCC1831
CAS No.:939983-14-9
- Synephrine
Catalog No.:BCN6308
CAS No.:94-07-5
- Benzocaine
Catalog No.:BCC4636
CAS No.:94-09-7
- Propylparaben
Catalog No.:BCN8416
CAS No.:94-13-3
- Sodium 4-amiropparaty Hyalrate
Catalog No.:BCC3855
CAS No.:94-16-6
- Benzyl 4-hydroxybenzoate
Catalog No.:BCC8869
CAS No.:94-18-8
Temporal and spatial dependence of inflammatory biomarkers and suppression by fluvastatin in dextran sodium sulfate-induced rat colitis model.[Pubmed:24781162]
Dig Dis Sci. 2014 Sep;59(9):2126-35.
BACKGROUND: Dextran sodium sulfate (DSS)-induced colitis in rats is widely used as an experimental model for elucidating the etiology of ulcerative colitis (UC) and developing its novel remedy. We investigated the temporal and spatial changes in inflammatory mediators such as tumor necrosis factor (TNF)-alpha and inducible nitric oxide synthase (iNOS) in the regions of rectum and distal colon and examined whether statins, which were designed to lower plasma cholesterol levels, influenced those mediators. METHODS: Colitis was induced in rats by oral administration of 5 % DSS for 5 days, followed by 2 % DSS for 10 days. 5 % DSS rats were treated with fluvastatin (20 mg/kg) concomitantly for 5 days. The expression of inflammatory mediators of a sequence of four regions in rectum (R) and distal colon (D0, D1, and D2) was determined by quantitative RT-PCR. RESULTS: The peak of colitic damage, which was confirmed clinically and histopathologically, was found on days 4-6. The expression of TNF-alpha, iNOS, cytokine-induced neutrophil chemoattractant-1, interleukin (IL)-1beta, and IL-6 mRNA increased in R time dependently, showing the peak on days 4-6, and then decreased thereafter. The levels of mRNAs reduced from R to D0, D1, and D2 region dependently. Fluvastatin decreased the expression of these markers in addition to the prevention of DSS-induced damage. CONCLUSIONS: Results demonstrated that the expression of inflammatory biomarkers had time and region specificity and was markedly inhibited by fluvastatin. To obtain a precise drug effect for UC, it is important to elucidate the temporal and spatial dependence of inflammatory biomarkers in DSS colitis model.
Simvastatin Sodium Salt and Fluvastatin Interact with Human Gap Junction Gamma-3 Protein.[Pubmed:26863535]
PLoS One. 2016 Feb 10;11(2):e0148266.
Finding pleiomorphic targets for drugs allows new indications or warnings for treatment to be identified. As test of concept, we applied a new chemical genomics approach to uncover additional targets for the widely prescribed lipid-lowering pro-drug simvastatin. We used mRNA extracted from internal mammary artery from patients undergoing coronary artery surgery to prepare a viral cardiovascular protein library, using T7 bacteriophage. We then studied interactions of clones of the bacteriophage, each expressing a different cardiovascular polypeptide, with surface-bound simvastatin in 96-well plates. To maximise likelihood of identifying meaningful interactions between simvastatin and vascular peptides, we used a validated photo-immobilisation method to apply a series of different chemical linkers to bind simvastatin so as to present multiple orientations of its constituent components to potential targets. Three rounds of biopanning identified consistent interaction with the clone expressing part of the gene GJC3, which maps to Homo sapiens chromosome 7, and codes for gap junction gamma-3 protein, also known as connexin 30.2/31.3 (mouse connexin Cx29). Further analysis indicated the binding site to be for the N-terminal domain putatively 'regulating' connexin hemichannel and gap junction pores. Using immunohistochemistry we found connexin 30.2/31.3 to be present in samples of artery similar to those used to prepare the bacteriophage library. Surface plasmon resonance revealed that a 25 amino acid synthetic peptide representing the discovered N-terminus did not interact with simvastatin lactone, but did bind to the hydrolysed HMG CoA inhibitor, simvastatin acid. This interaction was also seen for fluvastatin. The gap junction blockers carbenoxolone and flufenamic acid also interacted with the same peptide providing insight into potential site of binding. These findings raise key questions about the functional significance of GJC3 transcripts in the vasculature and other tissues, and this connexin's role in therapeutic and adverse effects of statins in a range of disease states.
Quantitative assessment of the contribution of sodium-dependent taurocholate co-transporting polypeptide (NTCP) to the hepatic uptake of rosuvastatin, pitavastatin and fluvastatin.[Pubmed:23996477]
Biopharm Drug Dispos. 2013 Nov;34(8):452-61.
Hepatic uptake transport is often the rate-determining step in the systemic clearance of drugs. The ability to predict uptake clearance and to determine the contribution of individual transporters to overall hepatic uptake is therefore critical in assessing the potential pharmacokinetic and pharmacodynamic variability associated with drug-drug interactions and pharmacogenetics. The present study revisited the interaction of statin drugs, including pitavastatin, fluvastatin and rosuvastatin, with the sodium-dependent taurocholate co-transporting polypeptide (NTCP) using gene transfected cell models. In addition, the uptake clearance and the contribution of NTCP to the overall hepatic uptake were assessed using in vitro hepatocyte models. Then NTCP protein expression was measured by a targeted proteomics transporter quantification method to confirm the presence and stability of NTCP expression in suspended and cultured hepatocyte models. It was concluded that NTCP-mediated uptake contributed significantly to active hepatic uptake in hepatocyte models for all three statins. However, the contribution of NTCP-mediated uptake to the overall active hepatic uptake was compound-dependent and varied from about 24% to 45%. Understanding the contribution of individual transporter proteins to the overall hepatic uptake and its functional variability when other active hepatic uptake pathways are interrupted could improve the current prediction practice used to assess the pharmacokinetic variability due to drug-drug interactions, pharmacogenetics and physiopathological conditions in humans.
Solid-state characterization and dissolution properties of Fluvastatin sodium salt hydrates.[Pubmed:23033850]
Pharm Dev Technol. 2013 Mar-Apr;18(2):525-34.
The present study reports the solid-state properties of Fluvastatin Sodium salt crystallized from different solvents for comparison with crystalline forms of the commercially available raw material and United States Pharmacopeia (USP) reference standard. Fluvastatin (FLV) samples were characterized by several techniques; such as X-ray powder diffractometry, differential scanning calorimetry, thermogravimetry, liquid and solid-state nuclear magnetic resonance spectroscopy, diffuse reflectance infrared Fourier transform spectroscopy, and scanning electron microscopy. In addition, intrinsic dissolution rate (IDR) of samples was performed in order to study the influence of crystalline form and other factors on rate and extent of dissolution. Three different forms were found. The commercial raw material and Fluvastatin-Acetonitrile (ACN) were identified as "form I" hydrate, the USP reference standard as "form II" hydrate and an ethanol solvate which presented a mixture of phases. Form I, with water content of 4%, was identified as monohydrate.
Comparison of the efficacies of five different statins on inhibition of human saphenous vein smooth muscle cell proliferation and invasion.[Pubmed:18049315]
J Cardiovasc Pharmacol. 2007 Oct;50(4):458-61.
Statins (HMG-CoA reductase inhibitors) exhibit beneficial effects on the vasculature independently of their cholesterol-lowering properties. These pleiotropic effects underlie the ability of statins to reduce intimal hyperplasia in saphenous vein (SV) bypass grafts by attenuating smooth muscle cell (SMC) invasion and proliferation. Although all statins can effectively lower cholesterol, the pleiotropic effects of individual statins may well differ. We therefore compared the concentration-dependent effects of 4 lipophilic statins (simvastatin, atorvastatin, fluvastatin, and lovastatin) and 1 hydrophilic statin (pravastatin) on the proliferation and invasion of SMC cultured from SV of 9 different patients undergoing coronary artery bypass grafting (CABG). The lipophilic statins inhibited SV-SMC proliferation over a 4-day period with an order of potency of fluvastatin > atorvastatin > simvastatin > lovastatin (IC50 range = 0.07 to 1.77 microM). Similarly, these statins also inhibited SV-SMC invasion through an artificial basement membrane barrier (fluvastatin > atorvastatin > simvastatin >> lovastatin; IC50 range = 0.92 to 26.9 microM). In contrast, the hydrophilic pravastatin had no significant effect on SV-SMC proliferation at concentrations up to 10 microM, nor did it attenuate SV-SMC invasion (up to 30 microM). Our data provide strong evidence that individual statins possess differential pleiotropic effects on SV-SMC function. This may be of clinical relevance in the selection of individual statins for the treatment of CABG patients.
Antioxidative effect of fluvastatin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, on peroxidation of phospholipid liposomes.[Pubmed:11273020]
J Pharm Pharmacol. 2001 Feb;53(2):227-32.
The antioxidative effect of Fluvastatin Sodium (fluvastatin), a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, on lipid peroxidation of phosphatidylcholine (PC) liposomes was investigated in various peroxidizing systems. Fluvastatin markedly inhibited the formation of thiobarbituric acid reactive substances in iron (II)-supported peroxidation of liposomes (IC50 = 1.2 x 10(-5) M). The order of magnitude of inhibition of each drug on the peroxidation was: butylated hydroxytoluene > fluvastatin > or = probucol >> pravastatin. Moreover, concentrations of fluvastatin ranging from 1 x 10(-6) to 1 x 10(-4) M inhibited peroxyl radical-mediated peroxidation of liposomes induced by water-soluble and lipid-soluble radical generators, 2,2'-azobis (2-amidinopropane) dihydro-chloride and 2,2'-azobis (2,4-dimethylvaleronitrile), respectively. However, pravastatin showed no effect against peroxyl radical-mediated peroxidation. These results indicate that fluvastatin acted non-enzymatically as an effective inhibitor against lipid peroxidation of PC liposomes and that the antioxidative effects of fluvastatin may be due to the scavenging action of fluvastatin on liposomal lipid peroxidation induced by peroxyl radicals generated in the aqueous and lipid phases.
HMG-CoA reductase activity in human liver microsomes: comparative inhibition by statins.[Pubmed:10965989]
Exp Toxicol Pathol. 2000 May;52(2):145-8.
The aim of this study was to compare a number of vastatins, HMG-CoA reductase inhibitors, in human liver microsomes. HMG-CoA reductase activity was four times lower than the activity in untreated rat liver microsomes. Vastatins could be classified in this in vitro assay in three classes both in human and rat microsomes: the first one including cerivastatin with an IC50 of 6 nM, the second one with atorvastatin and fluvastatin (IC50) between 40 and 100 nM) and the third one containing pravastatin, simvastatin and lovastatin (IC50 between 100 and 300 nM).


