ExoticinCAS# 13364-94-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13364-94-8 | SDF | Download SDF |
| PubChem ID | 389000 | Appearance | Yellow powder |
| Formula | C23H26O10 | M.Wt | 462.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
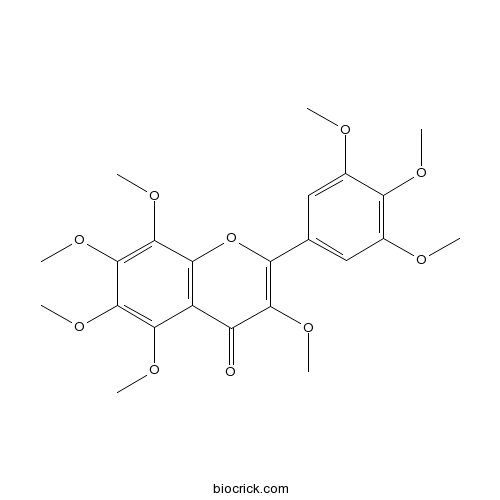
| Chemical Name | 3,5,6,7,8-pentamethoxy-2-(3,4,5-trimethoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC(=CC(=C1OC)OC)C2=C(C(=O)C3=C(O2)C(=C(C(=C3OC)OC)OC)OC)OC | ||
| Standard InChIKey | XOMNGQLSQXRGSF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H26O10/c1-25-12-9-11(10-13(26-2)17(12)27-3)16-20(29-5)15(24)14-18(28-4)21(30-6)23(32-8)22(31-7)19(14)33-16/h9-10H,1-8H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Exoticin shows antinociceptive and neuropharmacological activities, it could be considered as a suitable candidate for the development of analgesic and anxiolytic agents. |
| Animal Research | Polymethoxyflavones from Nicotiana plumbaginifolia (Solanaceae) exert antinociceptive and neuropharmacological effects in mice.[Reference: WebLink]Frontiers in Pharmacology, 2018, 9:85.Polymethoxylavones (PMFs) are known to exhibit significant anti-inflammatory and neuroprotective properties. Nicotiana plumbaginifolia, an annual Bangladeshi herb, is rich in polymethoxyflavones that possess significant analgesic and anxiolytic activities. The present study aimed to determine the antinociceptive and neuropharmacological activities of polyoxygenated flavonoids namely- 3,3′,5,6,7,8-hexamethoxy-4′,5′-methylenedioxyflavone (1), 3,3′,4′,5′,5,6,7,8-octamethoxyflavone (Exoticin) (2), 6,7,4′,5′-dimethylenedioxy-3,5,3′-trimethoxyflavone (3), and 3,3′,4′,5,5′,8-hexamethoxy-6,7-methylenedioxyflavone (4), isolated and identified from N. plumbaginifolia.
|

Exoticin Dilution Calculator

Exoticin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1622 mL | 10.8108 mL | 21.6216 mL | 43.2432 mL | 54.0541 mL |
| 5 mM | 0.4324 mL | 2.1622 mL | 4.3243 mL | 8.6486 mL | 10.8108 mL |
| 10 mM | 0.2162 mL | 1.0811 mL | 2.1622 mL | 4.3243 mL | 5.4054 mL |
| 50 mM | 0.0432 mL | 0.2162 mL | 0.4324 mL | 0.8649 mL | 1.0811 mL |
| 100 mM | 0.0216 mL | 0.1081 mL | 0.2162 mL | 0.4324 mL | 0.5405 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Aminoisonicotinic acid
Catalog No.:BCC8550
CAS No.:13362-28-2
- 2-Ethyl-2,6,6-trimethylpiperidin-4-one
Catalog No.:BCN6504
CAS No.:133568-79-3
- Fmoc-Ile-ol
Catalog No.:BCC2584
CAS No.:133565-46-5
- Boc-Threoninol(Bzl)
Catalog No.:BCC2704
CAS No.:133565-43-2
- Boc-Glutaminol
Catalog No.:BCC3092
CAS No.:133565-42-1
- Isoatriplicolide tiglate
Catalog No.:BCN7934
CAS No.:133559-39-4
- 4,15-Isoatriplicolide methylacrylate
Catalog No.:BCN7935
CAS No.:133559-38-3
- AG 556
Catalog No.:BCC6720
CAS No.:133550-41-1
- Tyrphostin B44, (+) enantiomer
Catalog No.:BCC6704
CAS No.:133550-37-5
- AG 494
Catalog No.:BCC6722
CAS No.:133550-35-3
- AG 555
Catalog No.:BCC6721
CAS No.:133550-34-2
- Tyrphostin B44, (-) enantiomer
Catalog No.:BCC6703
CAS No.:133550-32-0
- 1-Allyl-3,5-Dimethylpyrazole
Catalog No.:BCC8450
CAS No.:13369-74-9
- GSK 2193874
Catalog No.:BCC8009
CAS No.:1336960-13-4
- Ebenifoline E-II
Catalog No.:BCN3097
CAS No.:133740-16-6
- Suavioside A
Catalog No.:BCN6963
CAS No.:133740-37-1
- GSK2606414
Catalog No.:BCC1606
CAS No.:1337531-36-8
- GSK2656157
Catalog No.:BCC4983
CAS No.:1337532-29-2
- Dysolenticin J
Catalog No.:BCN7497
CAS No.:1337973-08-6
- Manninotriose
Catalog No.:BCN8488
CAS No.:13382-86-0
- LIMKi 3
Catalog No.:BCC7972
CAS No.:1338247-35-0
- SR 1664
Catalog No.:BCC6166
CAS No.:1338259-05-4
- Pseudolarolide Q2
Catalog No.:BCN8092
CAS No.:1338366-22-5
- EPZ004777
Catalog No.:BCC2218
CAS No.:1338466-77-5
Polymethoxyflavones from Nicotiana plumbaginifolia (Solanaceae) Exert Antinociceptive and Neuropharmacological Effects in Mice.[Pubmed:29515437]
Front Pharmacol. 2018 Feb 20;9:85.
Polymethoxylavones (PMFs) are known to exhibit significant anti-inflammatory and neuroprotective properties. Nicotiana plumbaginifolia, an annual Bangladeshi herb, is rich in polymethoxyflavones that possess significant analgesic and anxiolytic activities. The present study aimed to determine the antinociceptive and neuropharmacological activities of polyoxygenated flavonoids namely- 3,3',5,6,7,8-hexamethoxy-4',5'-methylenedioxyflavone (1), 3,3',4',5',5,6,7,8-octamethoxyflavone (Exoticin) (2), 6,7,4',5'-dimethylenedioxy-3,5,3'-trimethoxyflavone (3), and 3,3',4',5,5',8-hexamethoxy-6,7-methylenedioxyflavone (4), isolated and identified from N. plumbaginifolia. Antinociceptive activity was assessed using the acetic-acid induced writhing, hot plate, tail immersion, formalin and carrageenan-induced paw edema tests, whereas neuropharmacological effects were evaluated in the hole cross, open field and elevated plus maze test. Oral treatment of compounds 1, 3, and 4 (12.5-25 mg/kg b.w.) exhibited dose-dependent and significant (p < 0.01) antinociceptive activity in the acetic-acid, formalin, carrageenan, and thermal (hot plate)-induced pain models. The association of ATP-sensitive K(+) channel and opioid systems in their antinociceptive effect was obvious from the antagonist effect of glibenclamide and naloxone, respectively. These findings suggested central and peripheral antinociceptive activities of the compounds. Compound 1, 3, and 4 (12.5 mg/kg b.w.) demonstrated significant (p < 0.05) anxiolytic-like activity in the elevated plus-maze test, while the involvement of GABAA receptor in the action of compound 3 and 4 was evident from the reversal effects of flumazenil. In addition, compounds 1 and 4 (12.5-25 mg/kg b.w) exhibited anxiolytic activity without altering the locomotor responses. The present study suggested that the polymethoxyflavones (1-4) from N. Plumbaginifolia could be considered as suitable candidates for the development of analgesic and anxiolytic agents.


