Ecdysterone 2,3:20,22-diacetonideCAS# 22798-98-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22798-98-7 | SDF | Download SDF |
| PubChem ID | 11082278 | Appearance | Cryst. |
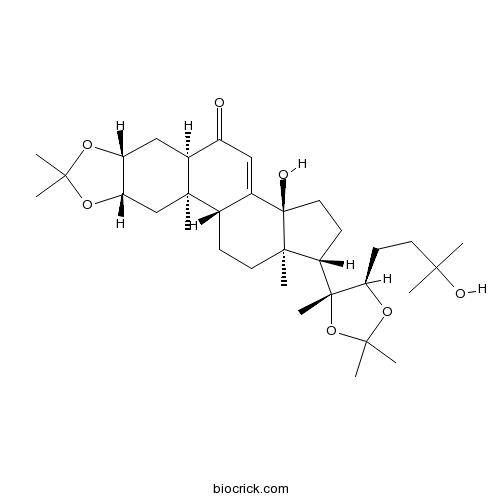
| Formula | C33H52O7 | M.Wt | 560.8 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2R,4S,8R,10R,14S,17S,18R)-14-hydroxy-17-[(4R,5R)-5-(3-hydroxy-3-methylbutyl)-2,2,4-trimethyl-1,3-dioxolan-4-yl]-2,6,6,18-tetramethyl-5,7-dioxapentacyclo[11.7.0.02,10.04,8.014,18]icos-12-en-11-one | ||
| SMILES | CC1(OC2CC3C(=O)C=C4C(C3(CC2O1)C)CCC5(C4(CCC5C6(C(OC(O6)(C)C)CCC(C)(C)O)C)O)C)C | ||
| Standard InChIKey | WXFMGCVRGSIXOB-APTIWFLNSA-N | ||
| Standard InChI | InChI=1S/C33H52O7/c1-27(2,35)13-12-26-32(9,40-29(5,6)39-26)25-11-15-33(36)20-16-22(34)21-17-23-24(38-28(3,4)37-23)18-30(21,7)19(20)10-14-31(25,33)8/h16,19,21,23-26,35-36H,10-15,17-18H2,1-9H3/t19-,21-,23+,24-,25-,26+,30+,31+,32+,33+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Due to the multi-drug resistance reversal activity of the less polar ecdysteroids, several new products( including 20-hydroxyecdysone,20-hydroxyecdysone 2,3;20,22-diacetonide) are promising for being tested against various cancer cell lines. |

Ecdysterone 2,3:20,22-diacetonide Dilution Calculator

Ecdysterone 2,3:20,22-diacetonide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7832 mL | 8.9158 mL | 17.8317 mL | 35.6633 mL | 44.5792 mL |
| 5 mM | 0.3566 mL | 1.7832 mL | 3.5663 mL | 7.1327 mL | 8.9158 mL |
| 10 mM | 0.1783 mL | 0.8916 mL | 1.7832 mL | 3.5663 mL | 4.4579 mL |
| 50 mM | 0.0357 mL | 0.1783 mL | 0.3566 mL | 0.7133 mL | 0.8916 mL |
| 100 mM | 0.0178 mL | 0.0892 mL | 0.1783 mL | 0.3566 mL | 0.4458 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ecdysterone 20,22-monoacetonide
Catalog No.:BCN5073
CAS No.:22798-96-5
- EPI-001
Catalog No.:BCC6536
CAS No.:227947-06-0
- Z-D-Trp-OH
Catalog No.:BCC2748
CAS No.:2279-15-4
- Ethyl 3-(4-methoxyphenyl)propanoate
Catalog No.:BCN4038
CAS No.:22767-72-2
- (±)-HIP-B
Catalog No.:BCC7295
CAS No.:227619-65-0
- (±)-HIP-A
Catalog No.:BCC7294
CAS No.:227619-64-9
- Rubroside H
Catalog No.:BCN1857
CAS No.:227597-43-5
- Rubroside G
Catalog No.:BCN1856
CAS No.:227597-42-4
- Xanthobaccin A
Catalog No.:BCN1864
CAS No.:227596-81-8
- Mucrolidin
Catalog No.:BCN5072
CAS No.:227471-20-7
- (3S,7S)-5,6-Dehydro-4''-de-O-methylcentrolobine
Catalog No.:BCN1481
CAS No.:227289-51-2
- AZ 11645373
Catalog No.:BCC7646
CAS No.:227088-94-0
- Ac-D-Trp-OH
Catalog No.:BCC3116
CAS No.:2280-01-5
- 1-Hydroxy-2,3,5-trimethoxyxanthone
Catalog No.:BCN6569
CAS No.:22804-49-5
- 1,2,3,7-Tetramethoxyxanthone
Catalog No.:BCN7519
CAS No.:22804-52-0
- 3-(4-Hydroxy-3,5-dimethoxyphenyl)-1,2-propanediol
Catalog No.:BCN1480
CAS No.:22805-15-8
- Alisol K 23-acetate
Catalog No.:BCN3363
CAS No.:228095-18-9
- Boc-Met-OH.DCHA
Catalog No.:BCC2602
CAS No.:22823-50-3
- Hypoglaunine A
Catalog No.:BCN3086
CAS No.:228259-16-3
- Miconazole nitrate
Catalog No.:BCC9047
CAS No.:22832-87-7
- Boc-D-Val-OH
Catalog No.:BCC3466
CAS No.:22838-58-0
- Aspartame
Catalog No.:BCC8836
CAS No.:22839-47-0
- Pratensein
Catalog No.:BCN2918
CAS No.:2284-31-3
- 9-Epiblumenol B
Catalog No.:BCN5075
CAS No.:22841-42-5
Rapid, laser-induced conversion of 20-hydroxyecdysone and its diacetonide -- experimental set-up of a system for photochemical transformation of bioactive substances.[Pubmed:22493361]
Anticancer Res. 2012 Apr;32(4):1291-7.
BACKGROUND: Photochemical transformation of certain bioactive compounds for the purpose of obtaining derivatives with increased bioactivity is a prospective area of synthetic chemistry. Ecdysteroids, analogs of the insect molting hormone, which can also exert several beneficial effects in mammals including humans, contain an enone moiety in their B ring, and, as such, are good candidates for photochemical transformations. MATERIALS AND METHODS: 20-hydroxyecdysone (20E), the most common ecdysteroid in Nature, and the easily obtained derivative 20-hydroxyecdysone 2,3;20,22-diacetonide (20ED), at different concentrations, were exposed to a 266 nm laser beam at an energy level of 6.5 mJ for different periods of time and evaluated for fluorescence emission during the process of irradiation. The products of irradiation were scanned from 200 to 1500 nm and then subjected to one-dimensional and two-dimensional thin layer chromatography. RESULTS: During irradiation, progressive significant changes in the fluorescence emission spectra were noted for both compounds with time that were accompanied by changes in their UV-Vis spectra. Full conversion of both compounds was reached within 14 minutes, and both compounds yielded several major products and several minor ones representing a wide polarity range. CONCLUSION: The photo-transformation system described here was proven to be a useful and flexibly adjustable tool for the laser-catalyzed conversion of bioactive compounds. Due to the multi-drug resistance reversal activity of the less polar ecdysteroids, several new products are promising for being tested against various cancer cell lines. Fractionation, isolation and characterization of the irradiated products are currently in process.


