EburicolCAS# 6890-88-6 |
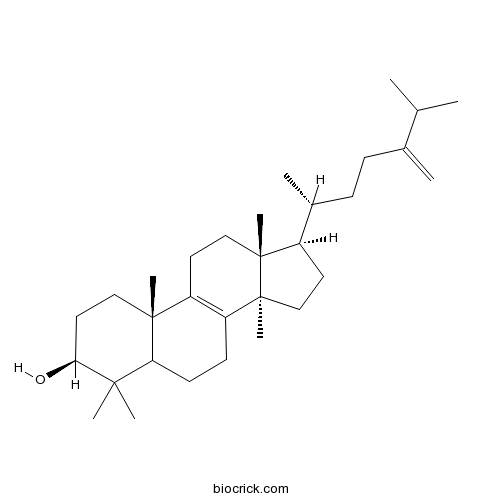
- Euphorbol
Catalog No.:BCN0321
CAS No.:566-14-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 6890-88-6 | SDF | Download SDF |
| PubChem ID | 65172 | Appearance | Powder |
| Formula | C31H52O | M.Wt | 440.8 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,10S,13R,14R,17R)-4,4,10,13,14-pentamethyl-17-[(2R)-6-methyl-5-methylideneheptan-2-yl]-2,3,5,6,7,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3-ol | ||
| SMILES | CC(C)C(=C)CCC(C)C1CCC2(C1(CCC3=C2CCC4C3(CCC(C4(C)C)O)C)C)C | ||
| Standard InChIKey | XJLZCPIILZRCPS-BGIAOWSUSA-N | ||
| Standard InChI | InChI=1S/C31H52O/c1-20(2)21(3)10-11-22(4)23-14-18-31(9)25-12-13-26-28(5,6)27(32)16-17-29(26,7)24(25)15-19-30(23,31)8/h20,22-23,26-27,32H,3,10-19H2,1-2,4-9H3/t22-,23-,26?,27+,29-,30-,31+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Eburicol inhibit the large accumulation of C-14 methyl sterols and cell lysis when the content of ergosterol becomes too low in the actively growing cells. 2. Mutations in the Eburicol gene impact on the activity of the Eburicol protein. |
| Targets | P450 (e.g. CYP17) |

Eburicol Dilution Calculator

Eburicol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2686 mL | 11.343 mL | 22.686 mL | 45.3721 mL | 56.7151 mL |
| 5 mM | 0.4537 mL | 2.2686 mL | 4.5372 mL | 9.0744 mL | 11.343 mL |
| 10 mM | 0.2269 mL | 1.1343 mL | 2.2686 mL | 4.5372 mL | 5.6715 mL |
| 50 mM | 0.0454 mL | 0.2269 mL | 0.4537 mL | 0.9074 mL | 1.1343 mL |
| 100 mM | 0.0227 mL | 0.1134 mL | 0.2269 mL | 0.4537 mL | 0.5672 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Otonecine
Catalog No.:BCN2009
CAS No.:6887-34-9
- 8-O-Methylretusin-7-O-beta-D-glucopyranoside
Catalog No.:BCN7899
CAS No.:68862-13-5
- HMP Linker
Catalog No.:BCC2832
CAS No.:68858-21-9
- Fmoc-Val-OH
Catalog No.:BCC3570
CAS No.:68858-20-8
- Boc-D-Tyr(Me)-OH
Catalog No.:BCC2597
CAS No.:68856-96-2
- Astemizole
Catalog No.:BCC7691
CAS No.:68844-77-9
- Xanthohumol L
Catalog No.:BCN8017
CAS No.:688360-15-8
- Xanthohumol I
Catalog No.:BCN8016
CAS No.:688360-06-7
- IWP 12
Catalog No.:BCC5622
CAS No.:688353-45-9
- 4-Epi-isoinuviscolide
Catalog No.:BCN4251
CAS No.:68832-39-3
- D-Prolinol(oil)
Catalog No.:BCC2708
CAS No.:68832-13-3
- Retusamine
Catalog No.:BCN2122
CAS No.:6883-16-5
- Guaiacol salicylate
Catalog No.:BCC8327
CAS No.:87-16-1
- H-D-Glu-OH
Catalog No.:BCC2936
CAS No.:6893-26-1
- Kahweol
Catalog No.:BCC9006
CAS No.:6894-43-5
- SKF 91488 dihydrochloride
Catalog No.:BCC6675
CAS No.:68941-21-9
- Mulberrofuran A
Catalog No.:BCN3677
CAS No.:68978-04-1
- Uncarine A
Catalog No.:BCN7767
CAS No.:6899-73-6
- Quinacrine 2HCl
Catalog No.:BCC4709
CAS No.:69-05-6
- Chlorpromazine HCl
Catalog No.:BCC4460
CAS No.:69-09-0
- Ampicillin
Catalog No.:BCC1199
CAS No.:69-52-3
- Penicillin G Sodium
Catalog No.:BCC4638
CAS No.:69-57-8
- D-Mannitol
Catalog No.:BCN2205
CAS No.:69-65-8
- Salicylic acid
Catalog No.:BCC4109
CAS No.:69-72-7
Cloning and sequence analysis of the eburicol 14alpha-demethylase gene of the obligate biotrophic grape powdery mildew fungus.[Pubmed:9300816]
Gene. 1997 Aug 11;195(1):29-33.
In order to obtain molecular data concerning field resistance of Uncinula necator, the causal agent of grape powdery mildew, to sterol demethylation inhibitors, a major group of fungicides, the gene encoding the target of these compounds (Eburicol 14alpha-demethylase) was cloned and sequenced from this obligately biotrophic phytopathogenic fungus. This single-copy gene encodes a 524 amino acid protein which displays high similarity to other known sterol 14alpha-demethylases (CYP51s). The coding sequence is interrupted by two short introns at positions identical to introns in Penicillium italicum CYP51, which is the only other known CYP51 gene in which introns have been identified. Intron excision was verified by cDNA sequencing.
Eburicol, lichesterol, ergosterol, and obtusifoliol from polyene antibiotic-resistant mutants of Candida albicans.[Pubmed:326362]
Can J Microbiol. 1977 Jun;23(6):751-4.
Two classes of polyene-resistant mutants were isolated from survivors of N-methyl-N'-nitro-N-nitrosoguanidine treatment of a wild-type Candida albicans. An analysis of the major sterols of one class revealed an accumulation of lichesterol and fecosterol while the other class accumulated Eburicol, obtusifoliol, and lanosterol with minor quantities of C28 sterols.
Sterol content analysis suggests altered eburicol 14alpha-demethylase (CYP51) activity in isolates of Mycosphaerella graminicola adapted to azole fungicides.[Pubmed:19459949]
FEMS Microbiol Lett. 2009 Jun;296(2):266-73.
The recent decline in the effectiveness of some azole fungicides in controlling the wheat pathogen Mycosphaerella graminicola has been associated with mutations in the CYP51 gene encoding the azole target, the Eburicol 14alpha-demethylase (CYP51), an essential enzyme of the ergosterol biosynthesis pathway. In this study, analysis of the sterol content of M. graminicola isolates carrying different variants of the CYP51 gene has revealed quantitative differences in sterol intermediates, particularly the CYP51 substrate Eburicol. Together with CYP51 gene expression studies, these data suggest that mutations in the CYP51 gene impact on the activity of the CYP51 protein.


