DihydroperaksineCAS# 16100-84-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 16100-84-8 | SDF | Download SDF |
| PubChem ID | 10903055 | Appearance | Powder |
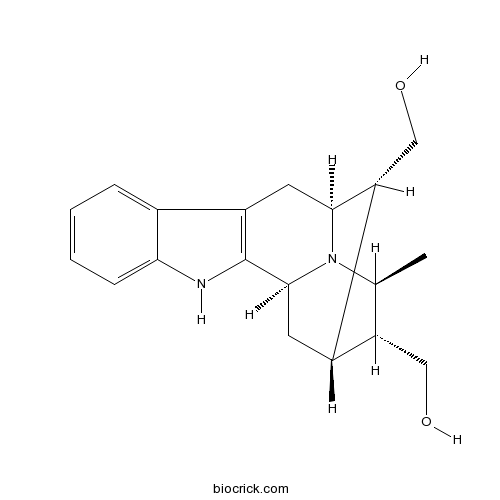
| Formula | C19H24N2O2 | M.Wt | 312.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1S,12S,13R,14S,15R,16S)-13-(hydroxymethyl)-16-methyl-3,17-diazapentacyclo[12.3.1.02,10.04,9.012,17]octadeca-2(10),4,6,8-tetraen-15-yl]methanol | ||
| SMILES | CC1C(C2CC3N1C(C2CO)CC4=C3NC5=CC=CC=C45)CO | ||
| Standard InChIKey | PBLXNPSLWYWTKM-KYGVLOPESA-N | ||
| Standard InChI | InChI=1S/C19H24N2O2/c1-10-14(8-22)12-6-18-19-13(7-17(21(10)18)15(12)9-23)11-4-2-3-5-16(11)20-19/h2-5,10,12,14-15,17-18,20,22-23H,6-9H2,1H3/t10-,12-,14-,15+,17-,18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | J Org Chem. 2014 Nov 7;79(21):10030-48.General strategy for synthesis of C-19 methyl-substituted sarpagine/macroline/ajmaline indole alkaloids including total synthesis of 19(S),20(R)-dihydroperaksine, 19(S),20(R)-dihydroperaksine-17-al, and peraksine.[Pubmed: 25247616]
Org Lett. 2011 Oct 7;13(19):5216-9.Regiospecific, enantiospecific total synthesis of C-19 methyl substituted sarpagine alkaloids dihydroperaksine-17-al and dihydroperaksine.[Pubmed: 21877687]
|

Dihydroperaksine Dilution Calculator

Dihydroperaksine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.201 mL | 16.0051 mL | 32.0102 mL | 64.0205 mL | 80.0256 mL |
| 5 mM | 0.6402 mL | 3.201 mL | 6.402 mL | 12.8041 mL | 16.0051 mL |
| 10 mM | 0.3201 mL | 1.6005 mL | 3.201 mL | 6.402 mL | 8.0026 mL |
| 50 mM | 0.064 mL | 0.3201 mL | 0.6402 mL | 1.2804 mL | 1.6005 mL |
| 100 mM | 0.032 mL | 0.1601 mL | 0.3201 mL | 0.6402 mL | 0.8003 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- TH588
Catalog No.:BCC6397
CAS No.:1609960-31-7
- TH287
Catalog No.:BCC6400
CAS No.:1609960-30-6
- Silodosin
Catalog No.:BCN2164
CAS No.:160970-54-7
- 2-[2-(2,2,2-Trifluoroethoxy)phenoxy]ethyl methanesulfonate
Catalog No.:BCN1546
CAS No.:160969-03-9
- 2-[2-(2,2,2-Trifluoroethoxy)phenoxy]ethyl bromide
Catalog No.:BCN1547
CAS No.:160969-00-6
- Mps1-IN-3
Catalog No.:BCC5496
CAS No.:1609584-72-6
- AZD3264
Catalog No.:BCC6514
CAS No.:1609281-86-8
- Villosin C
Catalog No.:BCN1712
CAS No.:160927-81-1
- Hosenkoside K
Catalog No.:BCN2577
CAS No.:160896-49-1
- Hosenkoside G
Catalog No.:BCN2272
CAS No.:160896-46-8
- Hosenkoside F
Catalog No.:BCN2520
CAS No.:160896-45-7
- Fmoc-Valinol
Catalog No.:BCC2694
CAS No.:160885-98-3
- Hosenkoside M
Catalog No.:BCN4518
CAS No.:161016-51-9
- VT-464
Catalog No.:BCC5398
CAS No.:1610537-15-9
- Epimedokoreanin B
Catalog No.:BCN6483
CAS No.:161068-53-7
- Epimedokoreanin C
Catalog No.:BCN8080
CAS No.:161068-54-8
- Formyl-DL-Trp-OH
Catalog No.:BCC3120
CAS No.:16108-03-5
- Cimicifugoside H2
Catalog No.:BCN7949
CAS No.:161097-77-4
- Bidwillol A
Catalog No.:BCN4858
CAS No.:161099-42-9
- H-Asp(OEt)-OEt.HCl
Catalog No.:BCC2888
CAS No.:16115-68-7
- Cimicidanol 3-O-alpha-L-arabinoside
Catalog No.:BCN6528
CAS No.:161207-05-2
- H-Orn(Z)-OtBu.HCl
Catalog No.:BCC2677
CAS No.:161234-80-6
- SR-9243
Catalog No.:BCC3983
CAS No.:1613028-81-1
- 1-O-Acetyl-6-O-isobutyrylbritannilactone
Catalog No.:BCN7795
CAS No.:1613152-34-3
General strategy for synthesis of C-19 methyl-substituted sarpagine/macroline/ajmaline indole alkaloids including total synthesis of 19(S),20(R)-dihydroperaksine, 19(S),20(R)-dihydroperaksine-17-al, and peraksine.[Pubmed:25247616]
J Org Chem. 2014 Nov 7;79(21):10030-48.
A detailed account of the development of a general strategy for synthesis of the C-19 methyl-substituted alkaloids including total synthesis of 19(S),20(R)-Dihydroperaksine-17-al (1), 19(S),20(R)-Dihydroperaksine (2), and peraksine (6) is presented. Efforts directed toward the total synthesis of macrosalhine chloride (5) are also reported. Important to success is the sequence of chemical reactions which include a critical haloboration reaction, regioselective hydroboration, and controlled oxidation (to provide sensitive enolizable aldehydes at C-20). In addition, the all-important Pd-catalyzed alpha-vinylation reaction has been extended to a chiral C-19 alkyl-substituted substrate for the first time. Synthesis of the advanced intermediate 64 completes an improved formal total synthesis of talcarpine (26) and provides a starting point for synthesis of macroline-related alkaloids 27-31. Similarly, extension of this synthetic strategy in the ring A oxygenated series should provide easy access to the northern hemisphere 32b of the bisindoles angustricraline, alstocraline, and foliacraline (Figure 4 ).
Regiospecific, enantiospecific total synthesis of C-19 methyl substituted sarpagine alkaloids dihydroperaksine-17-al and dihydroperaksine.[Pubmed:21877687]
Org Lett. 2011 Oct 7;13(19):5216-9.
The optically active tetracyclic ketone 8 was converted into the pentacylic core 14 of the C-19 methyl substituted N(a)-H sarpagine and ajmaline alkaloids via a critical haloboration reaction. The ketone 14 was then employed in the total synthesis of 19(S),20(R)-Dihydroperaksine-17-al (1) and 19(S),20(R)-Dihydroperaksine (2). The key regioselective hydroboration and controlled oxidation-epimerization sequence developed in this approach should provide a general method to functionalize the C(20)-C(21) double bond in the ajmaline-related indole alkaloids.
New alkaloids of the sarpagine group from Rauvolfia serpentina hairy root culture.[Pubmed:12141861]
J Nat Prod. 2002 Jul;65(7):1006-10.
Three new monoterpenoid indole alkaloids, 19(S),20(R)-Dihydroperaksine (1), 19(S),20(R)-Dihydroperaksine-17-al (2), and 10-hydroxy-19(S),20(R)-Dihydroperaksine (3), along with 16 known alkaloids 4-19 were isolated from hairy root culture of Rauvolfia serpentina, and their structures were elucidated by 1D and 2D NMR analyses. Taking into account the stereochemistry of the new alkaloids and results of preliminary enzymatical studies, the putative biosynthetical relationships between the novel alkaloids are discussed.


