DemethoxycapillarisinCAS# 61854-36-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 61854-36-2 | SDF | Download SDF |
| PubChem ID | 5316511 | Appearance | Powder |
| Formula | C15H10O6 | M.Wt | 286.24 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
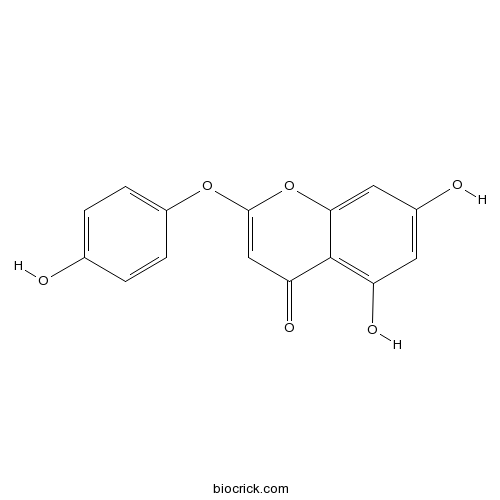
| Chemical Name | 5,7-dihydroxy-2-(4-hydroxyphenoxy)chromen-4-one | ||
| SMILES | C1=CC(=CC=C1O)OC2=CC(=O)C3=C(C=C(C=C3O2)O)O | ||
| Standard InChIKey | UBSCDKPKWHYZNX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O6/c16-8-1-3-10(4-2-8)20-14-7-12(19)15-11(18)5-9(17)6-13(15)21-14/h1-7,16-18H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Journal of the Chemical Society Chemical Communications, 1981, 12(10).Synthesis of demethoxycapillarisin, a naturally occurring 2-phenoxychromone, and related compounds.[Reference: WebLink]
|

Demethoxycapillarisin Dilution Calculator

Demethoxycapillarisin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4936 mL | 17.4679 mL | 34.9357 mL | 69.8714 mL | 87.3393 mL |
| 5 mM | 0.6987 mL | 3.4936 mL | 6.9871 mL | 13.9743 mL | 17.4679 mL |
| 10 mM | 0.3494 mL | 1.7468 mL | 3.4936 mL | 6.9871 mL | 8.7339 mL |
| 50 mM | 0.0699 mL | 0.3494 mL | 0.6987 mL | 1.3974 mL | 1.7468 mL |
| 100 mM | 0.0349 mL | 0.1747 mL | 0.3494 mL | 0.6987 mL | 0.8734 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Epoprostenol
Catalog No.:BCC7534
CAS No.:61849-14-7
- Benzyl p-coumarate
Catalog No.:BCN7717
CAS No.:61844-62-0
- Vorapaxar
Catalog No.:BCC3996
CAS No.:618385-01-6
- Sipeimine
Catalog No.:BCN1201
CAS No.:61825-98-7
- Oxaliplatin
Catalog No.:BCC3932
CAS No.:61825-94-3
- Trans-Melilotoside
Catalog No.:BCC8364
CAS No.:618-67-7
- Ethyl 3,4,5-trimethoxybenzoate
Catalog No.:BCN3973
CAS No.:6178-44-5
- 5-O-Methylnaringenin
Catalog No.:BCN4144
CAS No.:61775-19-7
- Methyl 2-(6-acetyl-5-hydroxy-2,3-dihydrobenzofuran-2-yl)propenoate
Catalog No.:BCN1395
CAS No.:617722-56-2
- Methyl 2-(5-acetyl-2,3-dihydrobenzofuran-2-yl)propenoate
Catalog No.:BCN1396
CAS No.:617722-55-1
- Fluvoxamine maleate
Catalog No.:BCC1215
CAS No.:61718-82-9
- W-7 hydrochloride
Catalog No.:BCC6622
CAS No.:61714-27-0
- Demethoxy-7-O-methylcapillarisin
Catalog No.:BCN6469
CAS No.:61854-37-3
- H-β-homo-Gln-OH.HCl
Catalog No.:BCC2648
CAS No.:61884-74-0
- Vernolide B
Catalog No.:BCN6749
CAS No.:618860-58-5
- 3-(1,1-Dimethylallyl)-8-hydroxy-7-methoxycoumarin
Catalog No.:BCN7570
CAS No.:61899-42-1
- 4-Hydroxybenzamide
Catalog No.:BCN4147
CAS No.:619-57-8
- p-Ethoxybenzoic acid
Catalog No.:BCN3378
CAS No.:619-86-3
- 4-Nitrocinnamic acid
Catalog No.:BCN5033
CAS No.:619-89-6
- Sceleratine
Catalog No.:BCN2126
CAS No.:6190-25-6
- Dihydroergotamine mesylate
Catalog No.:BCC5224
CAS No.:6190-39-2
- Tomatidine hydrochloride
Catalog No.:BCN2861
CAS No.:6192-62-7
- Boc-Cys(pMeBzl)-OH
Catalog No.:BCC3377
CAS No.:61925-77-7
- Deoxycalyciphylline B
Catalog No.:BCN4145
CAS No.:619326-74-8
Distinct Fractions of an Artemisia scoparia Extract Contain Compounds With Novel Adipogenic Bioactivity.[Pubmed:30906741]
Front Nutr. 2019 Mar 8;6:18.
Adipocytes are important players in metabolic health and disease, and disruption of adipocyte development or function contributes to metabolic dysregulation. Hence, adipocytes are significant targets for therapeutic intervention in obesity and metabolic syndrome. Plants have long been sources for bioactive compounds and drugs. In previous studies, we screened botanical extracts for effects on adipogenesis in vitro and discovered that an ethanolic extract of Artemisia scoparia (SCO) could promote adipocyte differentiation. To follow up on these studies, we have used various separation methods to identify the compound(s) responsible for SCO's adipogenic properties. Fractions and subfractions of SCO were tested for effects on lipid accumulation and adipogenic gene expression in differentiating 3T3-L1 adipocytes. Fractions were also analyzed by Ultra Performance Liquid Chromatography- Mass Spectrometry (UPLC-MS), and resulting peaks were putatively identified through high resolution, high mass accuracy mass spectrometry, literature data, and available natural products databases. The inactive fractions contained mostly quercetin derivatives and chlorogenates, including chlorogenic acid and 3,5-dicaffeoylquinic acid, which had no effects on adipogenesis when tested individually, thus ruling them out as pro-adipogenic bioactives in SCO. Based on these studies we have putatively identified the principal constituents in SCO fractions and subfractions that promoted adipocyte development and fat cell gene expression as prenylated coumaric acids, coumarin monoterpene ethers, 6-Demethoxycapillarisin and two polymethoxyflavones.
Qualitative variation of anti-diabetic compounds in different tarragon (Artemisia dracunculus L.) cytotypes.[Pubmed:21798321]
Fitoterapia. 2011 Oct;82(7):1062-74.
Ethanolic extracts of diploid Artemisia dracunculus L. (wild tarragon) from populations in the U.S., and polyploid tarragon from a variety of sources, were screened for the anti-diabetic compounds davidigenin; sakuranetin; 2',4'-dihydroxy-4-methoxydihydrochalcone; 4,5-di-O-caffeoylquinic acid; 5-O-caffeoylquinic acid and 6-Demethoxycapillarisin using LC-MS. Only decaploid plants contained all six target compounds and were the only plants that contained davidigenin and 2,4-dihydroxy-4-methoxydihydrochalcone. These results exhibit the importance of germplasm selection and provenance when studying plants for medicinal activity. Relying only on the "right species" for consistent medicinal activities may not be sufficient, as intraspecific variation may be highly significant.
Polyphenolic compounds from Artemisia dracunculus L. inhibit PEPCK gene expression and gluconeogenesis in an H4IIE hepatoma cell line.[Pubmed:17848630]
Am J Physiol Endocrinol Metab. 2007 Dec;293(6):E1503-10.
An ethanolic extract of Russian tarragon, Artemisia dracunculus L., with antihyperglycemic activity in animal models was reported to decrease phosphoenolpyruvate carboxykinase (PEPCK) mRNA expression in STZ-induced diabetic rats. A quantitative polymerase chain reaction (qPCR) assay was developed for the bioactivity-guided purification of the compounds within the extract that decrease PEPCK expression. The assay was based on the inhibition of dexamethasone-stimulated PEPCK upregulation in an H4IIE hepatoma cell line. Two polyphenolic compounds that inhibited PEPCK mRNA levels were isolated and identified as 6-Demethoxycapillarisin and 2',4'-dihydroxy-4-methoxydihydrochalcone with IC(50) values of 43 and 61 muM, respectively. The phosphoinositide-3 kinase (PI3K) inhibitor LY-294002 showed that 6-Demethoxycapillarisin exerts its effect through the activation of the PI3K pathway, similarly to insulin. The effect of 2',4'-dihydroxy-4-methoxydihydrochalcone is not regulated by PI3K and dependent on activation of AMPK pathway. These results indicate that the isolated compounds may be responsible for much of the glucose-lowering activity of the Artemisia dracunculus extract.
Bioassay-guided isolation of aldose reductase inhibitors from Artemisia dracunculus.[Pubmed:16806328]
Phytochemistry. 2006 Jul;67(14):1539-46.
An ethanolic extract of Artemisia dracunculus L. having antidiabetic activity was examined as a possible aldose reductase (ALR2) inhibitor, a key enzyme involved in diabetic complications. At 3.75 microg/mL, the total extract inhibited ALR2 activity by 40%, while quercitrin, a known ALR2 inhibitor, inhibited its activity by 54%. Bioactivity guided fractionation and isolation of the compounds that inhibit ALR2 activity was carried out with the total ethanolic extract yielding four bioactive compounds with ALR2 inhibitory activity ranging from 58% to 77% at 3.75 microg/mL. Using LC/MS, (1)H NMR, (13)C NMR and 2D NMR spectroscopic analyses, the four compounds were identified as 4,5-di-O-caffeoylquinic acid, davidigenin, 6-Demethoxycapillarisin and 2',4'-dihydroxy-4-methoxydihydrochalcone. This is the first report on their isolation from A. dracunculus and the ALR2 inhibitory activity of 4,5-di-O-caffeoylquinic acid, 6-Demethoxycapillarisin and 2',4'-dihydroxy-4-methoxydihydrochalcone. These results suggest a use of the extract of A. dracunculus for ameliorating diabetic complications.
Tenuiflorins A-C: new 2-phenoxychromones from the leaves of Mimosa tenuiflora.[Pubmed:15229805]
Planta Med. 2004 Jun;70(6):536-9.
Five 2-phenoxychromones, the new tenuiflorin A [5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenoxy)-6-methoxychromone], tenuiflorin B [5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenoxy)-6-methoxychromone] and tenuiflorin C [5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenoxy)chromone], along with the known 6-Demethoxycapillarisin and 6-demethoxy-4'- O-methylcapillarisin were isolated from leaves of Mimosa tenuiflora. Structures of these compounds were established by analysis of their spectroscopic data. A single-crystal diffraction analysis of 6-demethoxy-4'- O-methylcapillarisin was performed in order to confirm the proposed structures.


