Bisisorhapontigenin FCAS# N/A |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | N/A | SDF | Download SDF |
| PubChem ID | 11591617 | Appearance | Powder |
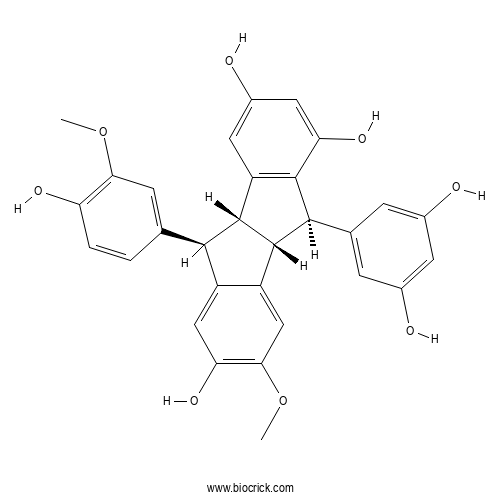
| Formula | C30H26O8 | M.Wt | 514.5 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4bR,5R,9bR,10R)-5-(3,5-dihydroxyphenyl)-10-(4-hydroxy-3-methoxyphenyl)-3-methoxy-4b,5,9b,10-tetrahydroindeno[2,1-a]indene-2,6,8-triol | ||
| SMILES | COC1=C(C=CC(=C1)C2C3C(C(C4=C3C=C(C=C4O)O)C5=CC(=CC(=C5)O)O)C6=CC(=C(C=C26)O)OC)O | ||
| Standard InChIKey | VCBDWSOYIDMSBG-BETQZWDCSA-N | ||
| Standard InChI | InChI=1S/C30H26O8/c1-37-24-7-13(3-4-21(24)34)26-18-11-22(35)25(38-2)12-19(18)29-27(14-5-15(31)8-16(32)6-14)28-20(30(26)29)9-17(33)10-23(28)36/h3-12,26-27,29-36H,1-2H3/t26-,27-,29+,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bisisorhapontigenin F Dilution Calculator

Bisisorhapontigenin F Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9436 mL | 9.7182 mL | 19.4363 mL | 38.8727 mL | 48.5909 mL |
| 5 mM | 0.3887 mL | 1.9436 mL | 3.8873 mL | 7.7745 mL | 9.7182 mL |
| 10 mM | 0.1944 mL | 0.9718 mL | 1.9436 mL | 3.8873 mL | 4.8591 mL |
| 50 mM | 0.0389 mL | 0.1944 mL | 0.3887 mL | 0.7775 mL | 0.9718 mL |
| 100 mM | 0.0194 mL | 0.0972 mL | 0.1944 mL | 0.3887 mL | 0.4859 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cyanidin 3-O-arabinoside
Catalog No.:BCN9100
CAS No.:792868-19-0
- α-Terpinene
Catalog No.:BCN9099
CAS No.:99-86-5
- Petunidin 3-O-glucoside
Catalog No.:BCN9098
CAS No.:71991-88-3
- Delphinidin 3-O-glucoside
Catalog No.:BCN9097
CAS No.:50986-17-9
- Malvidin 3-O-glucoside
Catalog No.:BCN9096
CAS No.:18470-06-9
- Febrifugine dihydrochloride
Catalog No.:BCN9095
CAS No.:32434-42-7
- Cyanidin 3-O- galactopyranoside
Catalog No.:BCN9094
CAS No.:142506-26-1
- Ephedrine
Catalog No.:BCN9093
CAS No.:299-42-3
- (5S,6S,7S,8R)-8-Chloro-5,6,7-trihydroxy-2-phenylethyl-5,6,7,8-tetrahydro-4H-chromen-4-one
Catalog No.:BCN9092
CAS No.:626236-06-4
- Bisabolone oxide A
Catalog No.:BCN9091
CAS No.:22567-38-0
- Bisisorhapontigenin E
Catalog No.:BCN9090
CAS No.:
- Delphinidin 3-O-galactoside
Catalog No.:BCN9089
CAS No.:197250-28-5
- 3-(2-Hydroxy-4,6-dimethoxyphenyl)-1-(4-hydroxyphenyl)-1-propanone
Catalog No.:BCN9102
CAS No.:151752-07-7
- (3R)-2,3-Dihydro-5,7-dihydroxy-3-[(4-hydroxyphenyl)methyl]-4H-1-benzopyran-4-one
Catalog No.:BCN9103
CAS No.:849727-88-4
- 4H-1-Benzopyran-4-one, 2,3-dihydro-3,5,7-trihydroxy-3-[(4-methoxyphenyl)methyl]-, (R)-
Catalog No.:BCN9104
CAS No.:118204-64-1
- Macrozamin
Catalog No.:BCN9105
CAS No.:6327-93-1
- (±)-Vasicine
Catalog No.:BCN9106
CAS No.:6159-56-4
- Loureirin D
Catalog No.:BCN9107
CAS No.:119425-91-1
- Bornyl Acetate
Catalog No.:BCN9108
CAS No.:76-49-3
- Epigambogic acid
Catalog No.:BCN9109
CAS No.:887606-04-4
- Rebaudioside N
Catalog No.:BCN9110
CAS No.:1220616-46-5
- Plantanone B
Catalog No.:BCN9111
CAS No.:55780-30-8
- Kaempferol-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside
Catalog No.:BCN9112
CAS No.:476617-49-9
- Kaempferol 3-O-rutinoside 7-O-glucoside
Catalog No.:BCN9113
CAS No.:34336-18-0
Effects of Biogenic Zinc Oxide Nanoparticles on Growth and Oxidative Stress Response in Flax Seedlings vs. In Vitro Cultures: A Comparative Analysis.[Pubmed:32560534]
Biomolecules. 2020 Jun 17;10(6). pii: biom10060918.
Linum usitatissimum biosynthesizes lignans and neolignans that are diet and medicinally valuable metabolites. In recent years, zinc oxide nanoparticles (ZnONPs) have emerged as potential elicitors for the enhanced biosynthesis of commercial secondary metabolites. Herein, we investigated the influence of biogenic ZnONPs on both seedlings and stem-derived callus of L. usitatissimum. Seedlings of L. usitatissimum grown on Murashige and Skoog (MS) medium supplemented with ZnONPs (1-1000 mg/L) presented the highest antioxidant activity, total phenolic content, total flavonoid content, peroxidase and superoxide dismutase activities at 500 mg/L, while the maximum plantlet length was achieved with 10 mg/L. Likewise, the high-performance liquid chromatography (HPLC) analysis revealed the enhanced production of secoisolariciresinol diglucoside, lariciresinol diglucoside, dehydrodiconiferyl alcohol glucoside and guaiacylglycerol-beta-coniferyl alcohol ether glucoside in the plantlets grown on the 500 mg/L ZnONPs. On the other hand, the stem explants were cultured on MS media comprising 1-naphthaleneacetic acid (1 mg/L) and ZnONPs (1-50 mg/L). The highest antioxidant and other activities with an enhanced rooting effect were noted in 25 mg/L ZnONP-treated callus. Similarly, the maximum metabolites were also accumulated in 25 mg/L ZnONP-treated callus. In both systems, the dose-dependent production of reactive oxygen species (ROS) was recorded, resulting in oxidative damage with a more pronounced toxic effect on in vitro cultures. Altogether, the results from this study constitute a first comprehensive view of the impact of ZnONPs on the oxidative stress and antioxidant responses in seedlings vs. in vitro cultures.
Feasible Production of Lignans and Neolignans in Root-derived In Vitro Cultures of Flax (Linum usitatissimum L.).[Pubmed:32218181]
Plants (Basel). 2020 Mar 25;9(4). pii: plants9040409.
Flax lignans and neolignans impart health benefits, particularly in treating different types of cancers, due to their strong phytoestrogenic and antioxidant properties. The present study enhances the comprehension on the biosynthesis of antioxidant lignans and neolignans in root-derived in vitro cultures of flax (both callus and adventitious root). The results presented here clearly showed that the adventitious root culture efficiently produced a higher amount of lignans (at day 40) and neolignans (at day 30) than callus culture of flax. High performance liquid chromatography (HPLC) analysis revealed that the accumulations of secoisolariciresinol diglucoside (SDG, 5.5 mg g(-)(1) DW (dry weight)) and dehydrodiconiferyl alcohol glucoside (DCG, 21.6 mg/g DW) were 2-fold higher, while guaiacylglycerol-beta-coniferyl alcohol ether glucoside (GGCG, 4.9 mg/g DW) and lariciresinol glucoside (LDG, 11.9 mg/g DW) contents were 1.5-fold higher in adventitious root culture than in callus culture. Furthermore, the highest level of total phenolic production (119.01 mg/L), with an antioxidant free radical scavenging activity of 91.01%, was found in adventitious root culture at day 40, while the maximum level of total flavonoid production (45.51 mg/L) was observed in callus culture at day 30 of growth dynamics. These results suggest that adventitious root culture can be a good candidate for scaling up to industrial level to commercially produce these pharmacologically and nutritionally valuable metabolites.
Diastereomeric identification of neolignan rhamnosides from Trochodendron aralioides leaves by LC-SPE-NMR and circular dichroism.[Pubmed:31857179]
Fitoterapia. 2020 Jul;144:104455.
Trochodendron aralioides is an old-existing relic plant with limited availability and only a few identified compounds. Accumulative analysis on the methanolic extract from its leaf part by LC-SPE-NMR resulted in the identification of seven new compounds, including three neolignan alpha-rhamnosides [(7R,8S)-dihydrodehydrodiconiferyl alcohol- 9-O-alpha-rhamnopyranoside (2) and 9'-O-alpha-rhamnopyranoside (3), and (7S,8R)-dehydrodiconiferyl alcohol-9'-O-alpha-rhamnopyranoside (4)], two isomeric oxyneolignan alpha-rhamnosides [(7R,8S)- (5) and (7R,8R)-icariside E8 (6)), and (7R,8S)- (10) and (7R,8R)-icariside E9 (11)], and two isomeric acylated fructofuranosyl mevalonolactones (13, 14), along with five known compounds (1, 7-9 and 12). The absolute configuration of the C-7 and C-8 positions for the new compounds 2-6 and 10-11 was assigned by comparison of the reported ECD spectra. Compounds 2, 3, 4, and 6 were further isolated by semi-preparative column chromatography for structure confirmation by 2D NMR spectroscopic analysis.
Dehydrodiconiferyl alcohol from Silybum marianum (L.) Gaertn accelerates wound healing via inactivating NF-kappaB pathways in macrophages.[Pubmed:31742713]
J Pharm Pharmacol. 2020 Feb;72(2):305-317.
OBJECTIVES: The aim of this study was to investigate the molecular mechanisms of the efficacy of lignin compound dehydrodiconiferyl alcohol (DHCA) isolated from Silybum marianum (L.) Gaertn in improving wound healing. These findings preliminarily brought to light the promising therapeutic potential of DHCA in skin wound healing. METHODS: First, the effect of DHCA on healing in vivo was studied using a full-thickness scalp wound model of mice by topical administration. Histopathological examinations were then conducted by haematoxylin and eosin (H&E), Masson's trichrome staining and the immunofluorescence assay. Second, we further examined the anti-inflammatory mechanism of DHCA in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages by immunofluorescence assay and Western blot analysis. KEY FINDINGS: DHCA could promote scalp wound healing in mice by enhancing epithelial cell proliferation and collagen formation and reducing inflammatory cells infiltration. Moreover, the NF-kappaB nuclear translocation was suppressed remarkably by DHCA administration in connective tissue of healing area. DHCA was also shown to inhibit production of nitric oxide (NO) and interleukin (IL)-1beta with downregulated inducible nitric oxide synthase (iNOS) expression in LPS-induced RAW 246.7 cells. More importantly, DHCA administration upregulated p-IkappaBalpha expression and induced nuclear translocation of NF-kappaB without affecting its expression. CONCLUSIONS: Our study indicated that DHCA exerted anti-inflammatory activity through inactivation of NF-kappaB pathways in macrophages and subsequently improved wound healing.
Biogenic zinc oxide nanoparticles-enhanced biosynthesis of lignans and neolignans in cell suspension cultures of Linum usitatissimum L.[Pubmed:31135228]
Artif Cells Nanomed Biotechnol. 2019 Dec;47(1):1367-1373.
Zinc oxide nanoparticles (NPs) have emerged as a novel elicitor for enhanced biosynthesis of secondary metabolites in in vitro plant cell cultures. The current study was aimed to explore elicitation abilities of ZnO-NPs for enhanced accumulation of lignans and neolignans in cell cultures of Linum usitatissimum. We optimized concentration of zinc oxide NPs before carrying out a full-fledged experiment. Subsequently, an optimum dose of 100 mg/l was introduced into the culture medium on day 0, days 0 and 15, and finally days 0 and 25. We observed that repeated elicitation stimulated various parameters and physiological responses in Linum usitatissimum cell cultures than one-time elicitation. Repeated elicitation of cell cultures on day 0 and 15 resulted in highest fresh weight (412.16 g/l) and lignans production (secoisolariciresinol diglucoside 284.12 mg/l: lariciresinol diglucoside 86.97 mg/l). Contrarily, repeated elicitation on day 0 and 25 resulted in highest DW (13.53 g/l), total phenolic production (537.44 mg/l), total flavonoid production (123.83 mg/l) and neolignans production (dehydrodiconiferyl alcohol glucoside 493.28 mg/l: guaiacylglycerol-beta-coniferyl alcohol ether glucoside 307.69 mg/l). Enhancement in plant growth and secondary metabolites accumulation was several fold higher than controls. Furthermore, a linear relationship existed between total phenolic and flavonoid contents which in turn was correlated with higher antioxidant activities.
An anti-inflammatory C-stiryl iridoid from Camptosorus sibiricus Rupr.[Pubmed:30880242]
Fitoterapia. 2019 Apr;134:378-381.
A new iridoid glycoside, named camptoside (1), together with three known compounds as dehydrodiconiferyl alcohol-9'-O-beta-d-glucopyranoside (2), aesculetin (3) and vajicoside (4), have been isolated from Camptosorus sibiricus Rupr. (Aspleniaceae). Their structures were established on the basis of spectroscopic analysis, especially 1D- and 2D-NMR data, and by comparison of their spectroscopic and physical data with those reported in the literature. Compounds 1-3 exhibited inhibitions of nitric oxide production in lipopolysaccharide-induced RAW 264.7 macrophages with IC50 values of 11.2, 8.3 and 9.4muM, respectively.
Salicylic acid-enhanced biosynthesis of pharmacologically important lignans and neo lignans in cell suspension culture of Linum ussitatsimum L.[Pubmed:32624999]
Eng Life Sci. 2018 Dec 20;19(3):168-174.
Linum usitatsimum L. (flax) is a perennial herb with magnitude of medicinal and commercial applications. In the present study, we investigated the effects of salicylic acid (SA) on biosynthesis of lignans (secoisolariciresinol diglucoside (SDG) and lariciresinol diglucoside (LDG)) and neolignans (dehydrodiconiferyl alcohol glucoside (DCG) and guaiacylglycerol-beta-coniferyl alcohol ether glucoside (GGCG)) in cell cultures of flax. Moderate concentration of SA (50 muM) enhanced biomass accumulation (10.98 g/L dry weight (DW)), total phenolic content (37.81 mg/g DW), and antioxidant potential (87.23%) to two-fold than their respective controls after 72 h of exposure. However, higher levels of total flavonoid content (5.32 mg/g DW) were noted after 48 h of exposure to 50 muM of SA. HPLC analyses revealed that 50 muM SA, significantly enhanced biosynthesis of SDG (7.95 mg/g DW), LDG (7.52 mg/g DW), DCG (54.90 mg/g DW), and GGCG (16.78 mg/g DW), which was almost 2.7, 1.8, 3.88, and 3.98 fold higher than their respective controls after 72 h of exposure time, respectively. These results indicated that moderate concentrations of SA had significant effects on biosynthesis and productivity of lignans and neolignans in cell culture of L. usitatissimum.
Silychristin derivatives conjugated with coniferylalcohols from silymarin and their pancreatic alpha-amylase inhibitory activity.[Pubmed:30445852]
Nat Prod Res. 2020 Mar;34(6):759-765.
Silymarin is a mixture of flavonolignans extracted from the fruit of Silybum marianum (milk thistle). The latter is used as a medicinal plant to treat liver and gallbladder disorders. Recently, silymarin has been investigated for its effects against diabetes mellitus, and shown to reduce serum levels of glucose in model animals and in clinical trials. This effect can be explained mainly by the protective effect of silymarin against pancreatic beta-cells, but the involvement of other mechanisms is possible. We demonstrated the alpha-amylase inhibitory activity of silymarin and investigated the components responsible for this effect. Two major flavonolignans, silibinin and silychristin, did not show inhibition against alpha-amylase, but two novel silychristin derivatives conjugated with dehydrodiconiferyl alcohol were isolated as the mildly inhibiting components of silymarin. Further analyses indicated the presence of various silychristin derivatives in silymarin that may act synergistically to show alpha-amylase inhibitory activity.[Formula: see text].
Influence of new effective allelochemicals on the distribution of Cleome arabica L. community in nature.[Pubmed:30445848]
Nat Prod Res. 2020 Mar;34(6):773-781.
New allelochemicals were identified through bio-guided fractionation from the ethyl acetate of seeds extracts, which was the most autotoxic compared to the other plant parts. Phytochemical investigation of the seeds extracts of C. arabica by spectroscopy analyses has led to identify two new dammarane type triterpenes (4 and 9), with nine known analogues (1 - 3, 5 - 8, 10 and 11), a new cucurbitane triterpene (12), acylated dehydrodiconiferyl alcohol (13), and three highly oxygenated flavonols (14-16). The most autotoxic compounds on germination and seedling growth were elucidated as dammarane type triterpenes. However, less autotoxic effect was recorded by an inhibition under 50% for most of the identified flavonoids. These results suggest that those autotoxic substances may be used as a new bio-herbicide that may contribute to manage the distribution of C. arabica in agronomic field.[Formula: see text].
In vitro cultures of Linum usitatissimum L.: Synergistic effects of mineral nutrients and photoperiod regimes on growth and biosynthesis of lignans and neolignans.[Pubmed:30145465]
J Photochem Photobiol B. 2018 Oct;187:141-150.
The multipurpose plant species Linum usitatissimum famous for producing linen fibre and containing valuable pharmacologically active polyphenols, has rarely been tested for it's in vitro biosynthesis potential of lignans and neolignans. The current study aims at the synergistic effects of mineral nutrients variation and different photoperiod treatments on growth kinetics and biomass accumulation in in vitro cultures of Linum usitatissimum. Both nutrient quality and quantity affected growth patterns, as cultures established on Gamborg B5 medium had comparatively long exponential phase compared to Murashige and Skoog medium, while growth was slow but steady until last phases of the culture on Schenk and Hildebrandt medium. Similarly, we observed that boron deficiency and nitrogen limitation in culture medium (Gamborg B5 medium) enhanced callus biomass (fresh weight 413g/l and dry weight 20.7g/l), phenolics production (667.60mg/l), and lignan content (secoisolariciresinol diglucoside 6.33 and lariciresinol diglucoside 5.22mg/g dry weight respectively) at 16/8h light and dark-week 4, while that of neolignans (dehydrodiconiferyl alcohol glucoside 44.42 and guaiacylglycerol-beta-coniferyl alcohol ether glucoside 9.26mg/g dry weight, respectively) in continuous dark after 4th week of culture. Conversely, maximum flavonoids production occurred at both Murashige and Skoog, Schenk and Hildebrandt media (both media types contain comparatively higher boron and nitrogen content) in the presence of continuous light. Generally, continuous dark had no significant role in any growth associated parameter. This study opens new dimension for optimizing growing conditions and evaluating underlying mechanisms in biosynthesis of lignans and neolignans in in vitro cultures of Linum usitatissimum.
[Study on lignans from Xanthii Fructus].[Pubmed:29933677]
Zhongguo Zhong Yao Za Zhi. 2018 May;43(10):2097-2103.
This project is to investigate lignans from the dried fruits of Xanthium sibiricum (Xanthii Fructus). The chemical constituents were extract by 70% ethanol and isolated by silica gel, ODS, Sephadex LH-20, MCI column chromatography. Based on comparison of their spectral data with those reported in literature, they were elucidated as (-)-pinoresinol (1), balanophonin A (2), diospyrosin (3), dehydrodiconiferyl alcohol (4), 2-(4-hydroxy-3-methoxyphenyl)-3-(2-hydroxy-5-methoxyphenyl)-3-oxo-1-propanol (5), (-)-simulanol (6), (-)-7R,8S-dehydrodiconiferyl alcohol (7), chushizisin E (8), dihydrodehydrodiconiferyl alcohol (9), 7R,8S-dihydrodehydrodiconiferyl alcohol 4-O-beta-D-glucopyranoside (10), erythro-1,2-bis(4-hydroxy-3-methoxyphenyl)-1,3-propanediol (11), leptolepisol D (12), 8-O-4' neolignan 4-O-beta-glucopyranoside (13), (-)-1-O-beta-D-glucopyranosyl-2-{2-methoxy-4-[1-(E)-propen-3-ol]phenoxyl}-propane -3-ol(14), 1-(4-hydroxy-3-methoxy)-phenyl-2-[4-(1,2,3-trihydroxypropyl)-2-methoxy]-phenoxy-1 ,3-propandiol (15), threo-dihydroxy dehydrodiconiferyl alcohol (16), (-)-(2R)-1-O-beta-D-glucopyranosyl-2-{2-methoxy-4-[(E)-formylviny1]phenoxyl} propane-3-ol (17). Compound 2-17 were isolated from the genus Xanthium for the first time. Compound 1 were isolated form Xanthii Fructus for the first time.
Dehydrodiconiferyl Alcohol Inhibits Osteoclast Differentiation and Ovariectomy-Induced Bone Loss through Acting as an Estrogen Receptor Agonist.[Pubmed:29869503]
J Nat Prod. 2018 Jun 22;81(6):1343-1356.
Estrogen deficiency after menopause increases bone loss by activating RANKL-induced osteoclast differentiation. Dehydrodiconiferyl alcohol (DHCA), a lignan originally isolated from Cucurbita moschata, has been thought to be a phytoestrogen based on its structure. In this study, we tested whether DHCA could affect RANKL-induced osteoclastogenesis in vitro and ovariectomy-induced bone loss in vivo. In RAW264.7 cells, DHCA inhibited RANKL-induced differentiation of osteoclasts. Consistently, expression of the six osteoclastogenic genes induced by RANKL was down-regulated. DHCA was also shown to suppress the NF-kappaB and p38 MAPK signaling pathways by activating AMPK. Data from transient transfection assays suggested that DHCA might activate the estrogen receptor signaling pathway. Effects of DHCA on RANKL-induced osteoclastogenesis were reduced when cells were treated with specific siRNA to ERalpha, but not to ERbeta. Interestingly, DHCA was predicted from molecular docking simulation to bind to both ERalpha and ERbeta. Indeed, data from an estrogen receptor competition assay revealed that DHCA acted as an agonist on both estrogen receptors. In the ovariectomized (Ovx) mouse model, DHCA prevented Ovx-induced bone loss by inhibiting osteoclastogenesis. Taken together, our results suggest that DHCA may be developed as an efficient therapeutic for osteoporosis by regulating osteoclastogenesis through its estrogenic effects.
Two novel decarboxylase genes play a key role in the stereospecific catabolism of dehydrodiconiferyl alcohol in Sphingobium sp. strain SYK-6.[Pubmed:29528542]
Environ Microbiol. 2018 May;20(5):1739-1750.
Sphingobium sp. strain SYK-6 is able to use a phenylcoumaran-type biaryl, dehydrodiconiferyl alcohol (DCA), as a sole source of carbon and energy. In SYK-6 cells, the alcohol group of the B-ring side chain of DCA was first oxidized to the carboxyl group, and then the alcohol group of the A-ring side chain was oxidized to generate 5-(2-carboxyvinyl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-2,3-dihydrobenzofuran- 3-carboxylate (DCA-CC). We identified phcF, phcG and phcH, which conferred the ability to convert DCA-CC into 3-(4-hydroxy-3-(4-hydroxy-3-methoxystyryl)-5-methoxyphenyl)acrylate (DCA-S) in a host strain. These genes exhibited no significant sequence similarity with known enzyme genes, whereas phcF and phcG, which contain a DUF3237 domain of unknown function, showed 32% amino acid sequence identity with each other. The DCA-CC conversion activities were markedly decreased by disruption of phcF and phcG, indicating that phcF and phcG play dominant roles in the conversion of DCA-CC. Purified PhcF and PhcG catalysed the decarboxylation of the A-ring side chain of DCA-CC, producing DCA-S, and showed enantiospecificity towards (+)- and (-)-DCA-CC respectively. PhcF and PhcG formed homotrimers, and their Km for DCA-CC were determined to be 84 muM and 103 muM, and Vmax were 307 mumolmin(-1) mg(-1) and 137 mumolmin(-1) mg(-1) respectively. In conclusion, PhcF and PhcG are enantiospecific decarboxylases involved in phenylcoumaran catabolism.
Dehydrodiconiferyl alcohol promotes BMP-2-induced osteoblastogenesis through its agonistic effects on estrogen receptor.[Pubmed:29253565]
Biochem Biophys Res Commun. 2018 Jan 15;495(3):2242-2248.
Estrogen deficiency results in an imbalance between the levels of bone-resorping osteoclasts and bone-forming osteoblasts, eventually leading to overall bone loss. Dehydrodiconiferyl alcohol (DHCA), a lignan compound originally isolated from Cucurbita moschata, has been shown to bind to estrogen receptor, and indeed exhibits various activities of estrogen, such as anti-inflammatory and anti-oxidative stress effects. In this study, we tested whether synthetic DHCA could affect the BMP-2-induced osteoblastogenesis in vitro. In MC3T3-E1 cells, DHCA promoted BMP-2-induced differentiation of osteoblasts. Consistently, the expression of three osteoblastogenic genes known to be induced by BMP-2, ALP, osteocalcin and OPG, was up-regulated by DHCA treatment. DHCA was also shown to activate the production of RUNX2 by activating Smad1/5/9 and AMPK. Data from transient transfection assays suggested that DHCA might activate the estrogen receptor signaling pathway. Effects of DHCA on BMP-2-induced osteoblastogenesis were reduced when cells were treated with a specific siRNA to ERalpha or ERbeta. Taken together, our results suggest that DHCA may be developed as an efficient therapeutic for osteoporosis by regulating osteoblastogenesis through its estrogenic effects.


