Daturataturin ACAS# 133360-51-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 133360-51-7 | SDF | Download SDF |
| PubChem ID | 14825627 | Appearance | Powder |
| Formula | C34H48O10 | M.Wt | 616.8 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
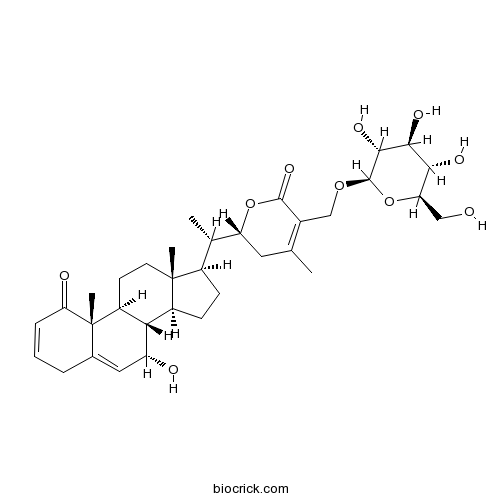
| Chemical Name | (2R)-2-[(1S)-1-[(7S,8S,9S,10R,13R,14S,17R)-7-hydroxy-10,13-dimethyl-1-oxo-4,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl]ethyl]-4-methyl-5-[[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]-2,3-dihydropyran-6-one | ||
| SMILES | CC1=C(C(=O)OC(C1)C(C)C2CCC3C2(CCC4C3C(C=C5C4(C(=O)C=CC5)C)O)C)COC6C(C(C(C(O6)CO)O)O)O | ||
| Standard InChIKey | FYXDMSFPWCORTF-UWOSJZGMSA-N | ||
| Standard InChI | InChI=1S/C34H48O10/c1-16-12-24(43-31(41)19(16)15-42-32-30(40)29(39)28(38)25(14-35)44-32)17(2)20-8-9-21-27-22(10-11-33(20,21)3)34(4)18(13-23(27)36)6-5-7-26(34)37/h5,7,13,17,20-25,27-30,32,35-36,38-40H,6,8-12,14-15H2,1-4H3/t17-,20+,21-,22-,23+,24+,25+,27-,28+,29-,30+,32+,33+,34-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | New anti-inflammatory withanolides from the leaves of Datura metel L.[Pubmed: 24844203]Steroids. 2014 Sep;87:26-34.
|
| Structure Identification | Chem Biodivers. 2006 Feb;3(2):180-6.Daturametelins H, I, and J: three new withanolide glycosides from Datura metel L.[Pubmed: 17193256]
|

Daturataturin A Dilution Calculator

Daturataturin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6213 mL | 8.1064 mL | 16.2127 mL | 32.4254 mL | 40.5318 mL |
| 5 mM | 0.3243 mL | 1.6213 mL | 3.2425 mL | 6.4851 mL | 8.1064 mL |
| 10 mM | 0.1621 mL | 0.8106 mL | 1.6213 mL | 3.2425 mL | 4.0532 mL |
| 50 mM | 0.0324 mL | 0.1621 mL | 0.3243 mL | 0.6485 mL | 0.8106 mL |
| 100 mM | 0.0162 mL | 0.0811 mL | 0.1621 mL | 0.3243 mL | 0.4053 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lydicamycin
Catalog No.:BCN1843
CAS No.:133352-27-9
- Lactacystin
Catalog No.:BCN1841
CAS No.:133343-34-7
- CHR-6494
Catalog No.:BCC1479
CAS No.:1333377-65-3
- Imbricataflavone A
Catalog No.:BCN8025
CAS No.:133336-96-6
- 10-Deacetylyunnanxane
Catalog No.:BCN7338
CAS No.:1333323-17-3
- TRPC6 inhibitor
Catalog No.:BCC4199
CAS No.:1333207-63-8
- KPT-185
Catalog No.:BCC4444
CAS No.:1333151-73-7
- GSK2194069
Catalog No.:BCC8053
CAS No.:1332331-08-4
- 4-O-Methylbutein
Catalog No.:BCN6677
CAS No.:13323-67-6
- Heliangin
Catalog No.:BCN6487
CAS No.:13323-48-3
- AM 92016 hydrochloride
Catalog No.:BCC6825
CAS No.:133229-11-5
- PI3k-delta inhibitor 1
Catalog No.:BCC1861
CAS No.:1332075-63-4
- DV 7028 hydrochloride
Catalog No.:BCC6202
CAS No.:133364-62-2
- Fmoc-His(MMt)-OH
Catalog No.:BCC3503
CAS No.:133367-33-6
- 4-Hydroxy-11,12,13-trinor-5-eudesmen-7-one
Catalog No.:BCN6627
CAS No.:133369-42-3
- Fmoc-β-Homo-D-Tyr(tBu)-OH
Catalog No.:BCC2620
CAS No.:133373-24-7
- Brandioside
Catalog No.:BCN6770
CAS No.:133393-81-4
- 3-MPPI
Catalog No.:BCC6705
CAS No.:133399-65-2
- (+)-Aflavazole
Catalog No.:BCN7339
CAS No.:133401-09-9
- MG-132
Catalog No.:BCC1227
CAS No.:133407-82-6
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- PF 1022A
Catalog No.:BCC8064
CAS No.:133413-70-4
- 3'-Geranyl-3-prenyl-2',4',5,7-tetrahydroxyflavone
Catalog No.:BCN1583
CAS No.:1334309-44-2
- Angophorol
Catalog No.:BCN3965
CAS No.:133442-54-3
New anti-inflammatory withanolides from the leaves of Datura metel L.[Pubmed:24844203]
Steroids. 2014 Sep;87:26-34.
Nine new withanolides, named daturafolisides A-I (1-9), along with six known compounds (22R)-27-hydroxy-7alpha-methoxy-1-oxowitha-3,5,24-trienolide-27-O-beta-d-glucopyr anoside, Daturataturin A, daturametelin J, daturataurin B, baimantuoluoside B, 12-deoxywithastramonolide (10-15), were isolated from the leaves of Datura metel L. The structures and absolute stereochemistry of these compounds were elucidated by means of spectroscopic methods including 1D and 2D NMR techniques, mass spectrometry and circular dichroism (CD) analyses. All isolates were evaluated for in vitro anti-inflammatory potential using LPS-stimulated RAW 264.7 murine macrophages. Among them, compounds 1, 2, 14, and 15 exhibited significant inhibition of nitrite production with values of IC50 at 20.9, 17.7, 17.8, and 18.4muM. Compounds 3, 4, 6, and 13 presented moderate inhibitory activities with values of IC50 at 59.0, 52.8, 71.2, and 53.1muM, while the rest compounds displayed weak suppressive effect.
Daturametelins H, I, and J: three new withanolide glycosides from Datura metel L.[Pubmed:17193256]
Chem Biodivers. 2006 Feb;3(2):180-6.
Three new withanolide glycosides named daturametelins H-J (1-3), together with two known ones, Daturataturin A (4) and 7,27-dihydroxy-1-oxowitha-2,5,24-trienolide (5), were isolated from the MeOH extract of the aerial parts of Datura metel L. (Solanaceae). Their structures were determined mainly by spectroscopic techniques including 2D-NMR (HMBC, HMQC, (1)H,(1)H-COSY, NOESY) and MS experiments. Compounds 1-5 were tested for their antiproliferative activity towards the human colorectal carcinoma (HCT-116) cell line. The nonglycosidic compound 5 exhibited the highest activity of the tested withanolides, with an IC(50) value of 3.2+/-0.2 microM (Table 3).


