3-MPPI5-HT1A ligand CAS# 133399-65-2 |
- CZC24832
Catalog No.:BCC1507
CAS No.:1159824-67-5
- PI3Kγ inhibitor 1
Catalog No.:BCC4180
CAS No.:1172118-03-4
- CAY10505
Catalog No.:BCC4990
CAS No.:1218777-13-9
- IC-87114
Catalog No.:BCC1161
CAS No.:371242-69-2
- AS-605240
Catalog No.:BCC2495
CAS No.:648450-29-7
- AS-252424
Catalog No.:BCC4988
CAS No.:900515-16-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 133399-65-2 | SDF | Download SDF |
| PubChem ID | 4013695 | Appearance | Powder |
| Formula | C23H25N5O3 | M.Wt | 419.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in DMSO with gentle warming | ||
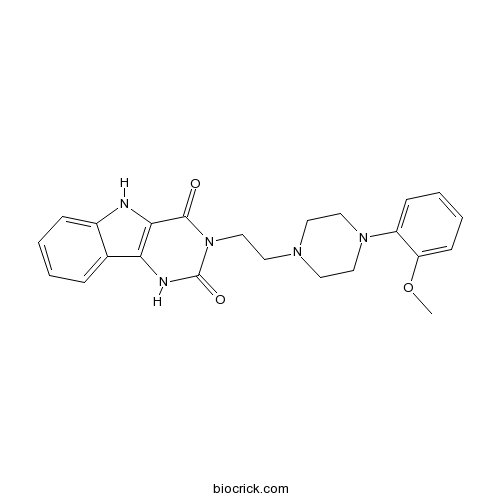
| Chemical Name | 3-[2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-1,5-dihydropyrimido[5,4-b]indole-2,4-dione | ||
| SMILES | COC1=CC=CC=C1N2CCN(CC2)CCN3C(=O)C4=C(C5=CC=CC=C5N4)NC3=O | ||
| Standard InChIKey | AQASGOHUMGAWJJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H25N5O3/c1-31-19-9-5-4-8-18(19)27-13-10-26(11-14-27)12-15-28-22(29)21-20(25-23(28)30)16-6-2-3-7-17(16)24-21/h2-9,24H,10-15H2,1H3,(H,25,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Very potent ligand for α1 sites (Ki for displacement of prazosin = 0.2 nM). Displays different binding properties for each α1 subtype (pKi values are 8.74, 9.44 and 9.57 for α1B, α1D and α1A adrenoceptors respectively). Also binds to 5-HT1A sites (Ki = 50 nM). |

3-MPPI Dilution Calculator

3-MPPI Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3839 mL | 11.9195 mL | 23.839 mL | 47.6781 mL | 59.5976 mL |
| 5 mM | 0.4768 mL | 2.3839 mL | 4.7678 mL | 9.5356 mL | 11.9195 mL |
| 10 mM | 0.2384 mL | 1.192 mL | 2.3839 mL | 4.7678 mL | 5.9598 mL |
| 50 mM | 0.0477 mL | 0.2384 mL | 0.4768 mL | 0.9536 mL | 1.192 mL |
| 100 mM | 0.0238 mL | 0.1192 mL | 0.2384 mL | 0.4768 mL | 0.596 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Brandioside
Catalog No.:BCN6770
CAS No.:133393-81-4
- Fmoc-β-Homo-D-Tyr(tBu)-OH
Catalog No.:BCC2620
CAS No.:133373-24-7
- 4-Hydroxy-11,12,13-trinor-5-eudesmen-7-one
Catalog No.:BCN6627
CAS No.:133369-42-3
- Fmoc-His(MMt)-OH
Catalog No.:BCC3503
CAS No.:133367-33-6
- DV 7028 hydrochloride
Catalog No.:BCC6202
CAS No.:133364-62-2
- Daturataturin A
Catalog No.:BCN6179
CAS No.:133360-51-7
- Lydicamycin
Catalog No.:BCN1843
CAS No.:133352-27-9
- Lactacystin
Catalog No.:BCN1841
CAS No.:133343-34-7
- CHR-6494
Catalog No.:BCC1479
CAS No.:1333377-65-3
- Imbricataflavone A
Catalog No.:BCN8025
CAS No.:133336-96-6
- 10-Deacetylyunnanxane
Catalog No.:BCN7338
CAS No.:1333323-17-3
- TRPC6 inhibitor
Catalog No.:BCC4199
CAS No.:1333207-63-8
- (+)-Aflavazole
Catalog No.:BCN7339
CAS No.:133401-09-9
- MG-132
Catalog No.:BCC1227
CAS No.:133407-82-6
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- PF 1022A
Catalog No.:BCC8064
CAS No.:133413-70-4
- 3'-Geranyl-3-prenyl-2',4',5,7-tetrahydroxyflavone
Catalog No.:BCN1583
CAS No.:1334309-44-2
- Angophorol
Catalog No.:BCN3965
CAS No.:133442-54-3
- Heudelotinone
Catalog No.:BCN6180
CAS No.:133453-58-4
- Iloperidone
Catalog No.:BCC2516
CAS No.:133454-47-4
- Fmoc-Glu(OAll)-OH
Catalog No.:BCC3492
CAS No.:133464-46-7
- TAI-1
Catalog No.:BCC5576
CAS No.:1334921-03-7
- SR 1001
Catalog No.:BCC6309
CAS No.:1335106-03-0
- Exendin-3 (9-39) amide
Catalog No.:BCC7257
CAS No.:133514-43-9
New pyrimido[5,4-b]indoles as ligands for alpha(1)-adrenoceptor subtypes.[Pubmed:12825930]
J Med Chem. 2003 Jul 3;46(14):2877-94.
A new series of compounds were designed as structural analogues of the alpha(1)-AR ligand RN5 (4), characterized by a tricyclic 5H-pyrimido[5,4-b]indole-(1H,3H)2,4-dione system connected through an alkyl chain to a phenylpiperazine (PP) moiety. These compounds were synthesized and tested in binding assays on human alpha(1A)-AR, alpha(1B)-AR, and alpha(1D)-AR subtypes expressed in HEK293 cells. Several structural modifications were performed on the PP moiety, the tricyclic system, and the connecting alkyl chain. Many of the new molecules showed a preferential affinity for the alpha(1D)-AR subtype. Some compounds, including 39 and 40, displayed substantial alpha(1D)-AR selectivity with respect to alpha(1A)-AR, alpha(1B)-AR, serotonergic 5-HT(1A), 5-HT(1B), 5-HT(2A), and dopaminergic D(1) and D(2) receptors. Two conformationally rigid analogues of 4, useful for studying the architecture of the receptor/ligand complex, were also prepared and tested. A subset of the new compounds was then used to evolve a preliminary pharmacophore model for alpha(1D)-AR antagonists, based on a more generalized model we had developed for alpha(1)-AR antagonists. This new model rationalized the relationships between structural properties and biological data of the pyrimido[5,4-b]indole compounds, as well as other compounds.
Pyrimido[5,4-b]indole derivatives. 1. A new class of potent and selective alpha 1 adrenoceptor ligands.[Pubmed:1648138]
J Med Chem. 1991 Jun;34(6):1850-4.
A number of 3-substituted pyrimido[5,4-b]indole-2,4-diones (7-23) were evaluated for their in vitro alpha 1 adrenoceptor affinity by radioligand receptor binding assays. Some compounds bearing a (phenylpiperazinyl)alkyl side chain were potent alpha 1 adrenoceptor ligands. The most active derivative in displacement of [3H]prazosin from rat cortical membranes was 3-[2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]pyrimido[5,4-b]indol e- 2,4-dione (10) (Ki = 0.21 nM). Discrete modifications in the structure resulted in higher selectivity (greater than 10,000 times) for alpha 1 than alpha 2, beta 2, and 5HT1A receptors. Some compounds also had affinity for the 5HT1A receptor. The most selective alpha 1 ligand was 3-[2-[4-(2-chlorophenyl)piperazin-1-yl]ethyl]pyrimido[5,4-b)indole - 2,4-dione (13).


