DatumetineCAS# 67078-20-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 67078-20-0 | SDF | Download SDF |
| PubChem ID | 181868 | Appearance | Powder |
| Formula | C16H21NO3 | M.Wt | 275.35 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
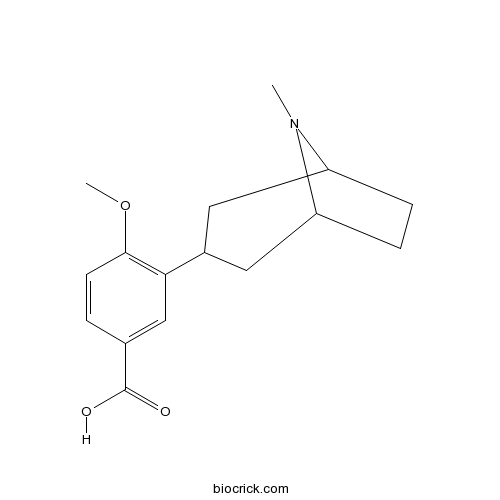
| Chemical Name | 4-methoxy-3-(8-methyl-8-azabicyclo[3.2.1]octan-3-yl)benzoic acid | ||
| SMILES | CN1C2CCC1CC(C2)C3=C(C=CC(=C3)C(=O)O)OC | ||
| Standard InChIKey | CMMJWJKGQZIJPB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H21NO3/c1-17-12-4-5-13(17)8-11(7-12)14-9-10(16(18)19)3-6-15(14)20-2/h3,6,9,11-13H,4-5,7-8H2,1-2H3,(H,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Phytochemistry 2005, 66(12):1448-1464.Chemotaxonomy of the pantropical genus Merremia (Convolvulaceae) based on the distribution of tropane alkaloids.[Reference: WebLink]
|

Datumetine Dilution Calculator

Datumetine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6317 mL | 18.1587 mL | 36.3174 mL | 72.6348 mL | 90.7935 mL |
| 5 mM | 0.7263 mL | 3.6317 mL | 7.2635 mL | 14.527 mL | 18.1587 mL |
| 10 mM | 0.3632 mL | 1.8159 mL | 3.6317 mL | 7.2635 mL | 9.0794 mL |
| 50 mM | 0.0726 mL | 0.3632 mL | 0.7263 mL | 1.4527 mL | 1.8159 mL |
| 100 mM | 0.0363 mL | 0.1816 mL | 0.3632 mL | 0.7263 mL | 0.9079 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Trichophydine
Catalog No.:BCN1781
CAS No.:67031-54-3
- Pepluanin A
Catalog No.:BCN4221
CAS No.:670257-89-3
- Ohchinin acetate
Catalog No.:BCN4220
CAS No.:67023-81-8
- Ohchinin
Catalog No.:BCN4601
CAS No.:67023-80-7
- Crenolanib (CP-868596)
Catalog No.:BCC3671
CAS No.:670220-88-9
- 3,4-Dihydroxy-2-methoxyxanthone
Catalog No.:BCN7631
CAS No.:6702-55-2
- Methyl isovanillate
Catalog No.:BCN7960
CAS No.:6702-50-7
- Anthriscusin
Catalog No.:BCN3533
CAS No.:67008-16-6
- Gliotoxin
Catalog No.:BCN3894
CAS No.:67-99-2
- Vitamin D3
Catalog No.:BCN2186
CAS No.:67-97-0
- Dicyclomine HCl
Catalog No.:BCC3762
CAS No.:67-92-5
- Fluocinolone Acetonide
Catalog No.:BCC4906
CAS No.:67-73-2
- Cyclo(Pro-Pro)
Catalog No.:BCN2415
CAS No.:6708-06-1
- Tubotaiwine
Catalog No.:BCN4016
CAS No.:6711-69-9
- Yamataimine
Catalog No.:BCN2064
CAS No.:67113-69-3
- Moronic acid
Catalog No.:BCN4222
CAS No.:6713-27-5
- (±)-U-50488 hydrochloride
Catalog No.:BCC6665
CAS No.:67197-96-0
- H-HoSer-OH
Catalog No.:BCC3247
CAS No.:672-15-1
- SMER 3
Catalog No.:BCC6152
CAS No.:67200-34-4
- 2'-O-Coumaroyljuglanin
Catalog No.:BCN6559
CAS No.:67214-05-5
- 5'-O-Dimethoxytrityl-N-benzoyl-desoxycytidine
Catalog No.:BCC8750
CAS No.:67219-55-0
- Fenoldopam
Catalog No.:BCC4462
CAS No.:67227-56-9
- 10(14)-Cadinene-4,5-diol
Catalog No.:BCN4223
CAS No.:672336-50-4
- Boc-MLF
Catalog No.:BCC6061
CAS No.:67247-12-5
Chemotaxonomy of the pantropical genus Merremia (Convolvulaceae) based on the distribution of tropane alkaloids.[Pubmed:15922373]
Phytochemistry. 2005 Jun;66(12):1448-64.
The occurrence and distribution of tropane and biogenetically related pyrrolidine alkaloids in 18 Merremia species of paleo-, neo-, and pantropical occurrence have been studied. The extensive GC-MS study included members of almost all sections of the genus and has been carried out with epigeal vegetative parts as well as with roots. It comprises altogether 74 tropanes and 13 pyrrolidines including nicotine. Along with Datumetine known already from a solanaceous species, the study led to the isolation (from M. dissecta and M. guerichii, respectively) and structure elucidation (spectral data) of four novel 3alpha-acyloxytropanes, merresectines A-D: 3alpha-(4-methoxybenzoyloxy)nortropane (A), 3alpha-kurameroyloxytropane (B), 3alpha-nervogenoyloxytropane (C), 3alpha-[4-(beta-D-glucopyranosyloxy)-3-methoxy-5-(3-methyl-2-butenyl)benzoyloxy]t ropane (beta-d-glucoside of D). Moreover, the novel 3alpha,6beta-di-(4-methoxybenzoyloxy)tropane (merredissine) has been isolated from M. dissecta and structurally elucidated. In addition the structures of Datumetine and merresectine A could be confirmed by synthesis. Spectral data for two known 3alpha-acyloxytropanes (merresectine E beta-D-glucoside, 4'-dihydroconsabatine) and one known 3beta-acyloxytropane (concneorine) are documented for the first time. The structures of three further merresectines (F-H) have been determined by mass spectrometry. Furthermore, the linkage (2',3- and 2',4-, respectively) of two position isomer N-methylpyrrolidinylhygrines was proven by synthesis. The results of the study contribute to the solution of infrageneric taxonomic problems. Whereas all species yield pyrrolidine alkaloids without suitably differentiating results the diverging occurrence of tropane alkaloids leads to three groups of sections: (1) taxa free of tropanes, (2) taxa with simple tropanes, and (3) taxa with merresectines in addition to simple tropanes.


