DMPOCAS# 3317-61-1 |
- ML347
Catalog No.:BCC5331
CAS No.:1062368-49-3
- LDN-212854
Catalog No.:BCC5330
CAS No.:1432597-26-6
- PD 169316
Catalog No.:BCC3969
CAS No.:152121-53-4
- Imperatorin
Catalog No.:BCN5574
CAS No.:482-44-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3317-61-1 | SDF | Download SDF |
| PubChem ID | 1774 | Appearance | Powder |
| Formula | C6H11NO | M.Wt | 113.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
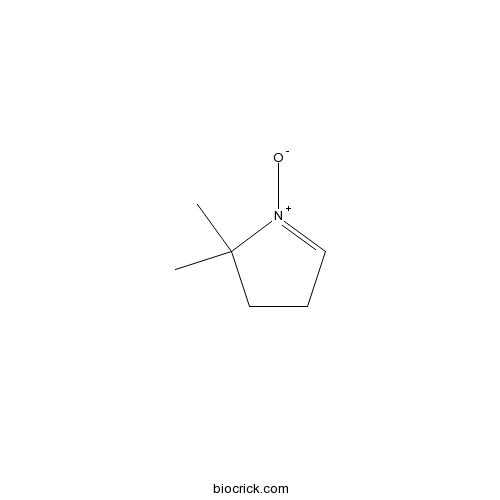
| Chemical Name | 2,2-dimethyl-1-oxido-3,4-dihydropyrrol-1-ium | ||
| SMILES | CC1(CCC=[N+]1[O-])C | ||
| Standard InChIKey | VCUVETGKTILCLC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H11NO/c1-6(2)4-3-5-7(6)8/h5H,3-4H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Water soluble nitric oxide spin trap; allows the measurement of oxygen-centered free radicals in biological systems at room temperature using electron spin resonance (ESR). Has a high reaction rate constant for superoxide and hydroxyl radicals, and distinguishes simultaneously among a variety of important biologically generated free radicals. |

DMPO Dilution Calculator

DMPO Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.837 mL | 44.1852 mL | 88.3704 mL | 176.7409 mL | 220.9261 mL |
| 5 mM | 1.7674 mL | 8.837 mL | 17.6741 mL | 35.3482 mL | 44.1852 mL |
| 10 mM | 0.8837 mL | 4.4185 mL | 8.837 mL | 17.6741 mL | 22.0926 mL |
| 50 mM | 0.1767 mL | 0.8837 mL | 1.7674 mL | 3.5348 mL | 4.4185 mL |
| 100 mM | 0.0884 mL | 0.4419 mL | 0.8837 mL | 1.7674 mL | 2.2093 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- CFM 4
Catalog No.:BCC8017
CAS No.:331458-02-7
- trans-2,3,4-Trimethoxycinnamic acid
Catalog No.:BCN5035
CAS No.:33130-03-9
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Boc-D-Phg-OH
Catalog No.:BCC3315
CAS No.:33125-05-2
- LG 101506
Catalog No.:BCC7696
CAS No.:331248-11-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Stephavanine
Catalog No.:BCN5253
CAS No.:33116-33-5
- PT 1
Catalog No.:BCC7846
CAS No.:331002-70-1
- IQ 1
Catalog No.:BCC7965
CAS No.:331001-62-8
- Caffeic acid
Catalog No.:BCN5979
CAS No.:331-39-5
- AS 1269574
Catalog No.:BCC7878
CAS No.:330981-72-1
- Bisdemethoxycurcumin
Catalog No.:BCN5975
CAS No.:33171-05-0
- 10-Demethoxy-10-(diethylamino)colchicine
Catalog No.:BCC8164
CAS No.:6962-03-4
- ZM 447439
Catalog No.:BCC2169
CAS No.:331771-20-1
- 2-(N,N-Dimethylamino)acetophenone
Catalog No.:BCN1747
CAS No.:3319-03-7
- 2',4'-Dihydroxy-7-methoxy-8-prenylflavan
Catalog No.:BCN6844
CAS No.:331954-16-6
- I2906
Catalog No.:BCC1637
CAS No.:331963-29-2
- Nτ-Methyl-His-OH
Catalog No.:BCC2957
CAS No.:332-80-9
- Telatinib (BAY 57-9352)
Catalog No.:BCC3879
CAS No.:332012-40-5
- H-Ala-NH2.HCl
Catalog No.:BCC2688
CAS No.:33208-99-0
- TCS 5861528
Catalog No.:BCC7816
CAS No.:332117-28-9
- Glucosyringic acid
Catalog No.:BCN5254
CAS No.:33228-65-8
- DZ2002
Catalog No.:BCC5544
CAS No.:33231-14-0
Identification of the transition state for fast reactions: the trapping of hydroxyl and methyl radicals by DMPO-A DFT approach.[Pubmed:25000097]
J Mol Graph Model. 2014 Jul;52:57-70.
Up to date, attempts to locate the transition state (TS) for the trapping reaction between OH and DMPO have been unsuccessful, and the lack of molecular mechanisms by which OH binds to the spin-trap constitutes a question still unsolved. Herein, we have taken a step forward on this task by describing the theoretical TS for the trapping of OH and CH3 by DMPO and the intrinsic reaction coordinates. This work aims to provide new understandings on the molecular orbital (MO) interactions that rule these reaction paths. Besides we assessed the degree of involvement of weak interactions and the charge transfer (CT) phenomenon involved in such interactions. Regarding the trapping of OH, the beginning of the reaction would be ruled by weak interactions to then give way to stronger MO interactions conducive to the formation of the TS. For CH3, the reaction is, instead, early ruled by significant MO interactions, and a relatively small contribution in the weak interactions range. At the TS, both reactions share the formation of an antibonding orbital responsible for hosting the unpaired electron, and two bonding orbitals between the radical and the spin-trap. Additionally, the charge is transferred primarily from DMPO to OH through beta orbitals, while for CH3, the CT occurs in both directions, so that while DMPO behaves like an alpha-acceptor/beta-donor, CH3 acts as a beta-acceptor/alpha-donor. Finally, we provide evidence showing that the resultant theoretical models are in agreement with the hyperfine coupling constants as obtained from biological-ESR spin trapping experiments.
Reversal of SIN-1-induced eNOS dysfunction by the spin trap, DMPO, in bovine aortic endothelial cells via eNOS phosphorylation.[Pubmed:24405159]
Br J Pharmacol. 2014 May;171(9):2321-34.
BACKGROUND AND PURPOSE: Nitric oxide (NO) derived from eNOS is mostly responsible for the maintenance of vascular homeostasis and its decreased bioavailability is characteristic of reactive oxygen species (ROS)-induced endothelial dysfunction (ED). Because 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), a commonly used spin trap, can control intracellular nitroso-redox balance by scavenging ROS and donating NO, it was employed as a cardioprotective agent against ED but the mechanism of its protection is still not clear. This study elucidated the mechanism of protection by DMPO against SIN-1-induced oxidative injury to bovine aortic endothelial cells (BAEC). EXPERIMENTAL APPROACH: BAEC were treated with SIN-1, as a source of peroxynitrite anion (ONOO(-)), and then incubated with DMPO. Cytotoxicity following SIN-1 alone and cytoprotection by adding DMPO was assessed by MTT assay. Levels of ROS and NO generation from HEK293 cells transfected with wild-type and mutant eNOS cDNAs, tetrahydrobiopterin bioavailability, eNOS activity, eNOS and Akt kinase phosphorylation were measured. KEY RESULTS: Post-treatment of cells with DMPO attenuated SIN-1-mediated cytotoxicity and ROS generation, restoration of NO levels via increased in eNOS activity and phospho-eNOS levels. Treatment with DMPO alone significantly increased NO levels and induced phosphorylation of eNOS Ser(1)(1)(7)(9) via Akt kinase. Transfection studies with wild-type and mutant human eNOS confirmed the dual role of eNOS as a producer of superoxide anion (O(2)(-)) with SIN-1 treatment, and a producer of NO in the presence of DMPO. CONCLUSION AND IMPLICATIONS: Post-treatment with DMPO of oxidatively challenged cells reversed eNOS dysfunction and could have pharmacological implications in the treatment of cardiovascular diseases.
Synthesis and spin-trapping properties of a trifluoromethyl analogue of DMPO: 5-methyl-5-trifluoromethyl-1-pyrroline N-oxide (5-TFDMPO).[Pubmed:24590621]
Chemistry. 2014 Apr 1;20(14):4064-71.
The 5-diethoxyphosphonyl-5-methyl-1-pyrroline N-oxide superoxide spin adduct (DEPMPO-OOH) is much more persistent (about 15 times) than the 5,5-dimethyl-1-pyrroline N-oxide superoxide spin adduct (DMPO-OOH). The diethoxyphosphonyl group is bulkier than the methyl group and its electron-withdrawing effect is much stronger. These two factors could play a role in explaining the different half-lifetimes of DMPO-OOH and DEPMPO-OOH. The trifluoromethyl and the diethoxyphosphonyl groups show similar electron-withdrawing effects but have different sizes. We have thus synthesized and studied 5-methyl-5-trifluoromethyl-1-pyrroline N-oxide (5-TFDMPO), a new trifluoromethyl analogue of DMPO, to compare its spin-trapping performance with those of DMPO and DEPMPO. 5-TFDMPO was prepared in a five-step sequence by means of the Zn/AcOH reductive cyclization of 5,5,5-trifluoro-4-methyl-4-nitropentanal, and the geometry of the molecule was estimated by using DFT calculations. The spin-trapping properties were investigated both in toluene and in aqueous buffer solutions for oxygen-, sulfur-, and carbon-centered radicals. All the spin adducts exhibit slightly different fluorine hyperfine coupling constants, thereby suggesting a hindered rotation of the trifluoromethyl group, which was confirmed by variable-temperature EPR studies and DFT calculations. In phosphate buffer at pH 7.4, the half-life of 5-TFDMPOOOH is about three times shorter than for DEPMPO-OOH and five times longer than for DMPO-OOH. Our results suggest that the stabilization of the superoxide adducts comes from a delicate balance between steric, electronic, and hydrogen-bonding effects that involve the beta group, the hydroperoxyl moiety, and the nitroxide.
An empirical method for automatic determination of maximum number of segments in DMPO-based IMRT for Head and Neck cases.[Pubmed:27721672]
Rep Pract Oncol Radiother. 2016 Nov-Dec;21(6):571-578.
AIM: An empirical scheme called "anatomy-guided segment counting (AGSC)" is proposed for automatic selection of maximum number of segments (NOS) for direct machine parameter optimization (DMPO). BACKGROUND: Direct machine parameter optimization (DMPO) requires the user to define the maximum number of segments (NOS) in order to proceed with an optimization process. Till date there is no established approach to arrive at an optimal and case-specific maximum NOS in DMPO, and this step is largely left to the planner's experience. MATERIALS AND METHODS: The AGSC scheme basically uses the Beam's-eye views (BEVs) and other planning parameters to decide on appropriate number of segments for the beam. The proposed algorithm was tested in eight H&N cases. We used Auto Plan feature available in Pinnacle3 (version 9.10.0) for driving the DMPO optimization. RESULTS: There is about 13% reduction in the composite objective value in AGSC plans as compared to the plans employing 6 NOS per beam and 10% increase in the composite objective value in AGSC plans as compared to the plans employing 8 NOS per beam. On the delivery efficiency front, there is about 10% increase in NOS in AGSC plans as compared to the plans employing 6 NOS per beam specification. Similarly, there is about 19% reduction in NOS in AGSC plans as compared to the plans employing 8 NOS per beam specification. CONCLUSION: The study demonstrates that the AGSC method allows specifying appropriate number of segments into the DMPO module accounting for the complexity of a given case.
The generation of lucigenin chemiluminescence from the reaction of guanidino compounds with phenylglyoxal under alkaline conditions and its application.[Pubmed:19571414]
Chem Pharm Bull (Tokyo). 2009 Jul;57(7):700-3.
It is shown that o-carboxyphenylglyoxal, which is converted from ninhydrin by alkali, produces a chemiluminescent lucigenin reaction under alkaline conditions when with reacted with guanidino compounds. It is also demonstrated that phenylglyoxal, which is a model compound of o-carboxyphenylglyoxal, produces a strong chemiluminescent lucigenin reaction under alkaline conditions when reacted with guanidino compounds. Moreover, ESR spectra showed the presence of 5,5-dimethyl-1-pyrroline N-oxide (DMPO)-spin adducts of superoxide anions in a mixture of phenylglyoxal and guanidino compounds under alkaline conditions. It was confirmed that the superoxide anions were generated by the reaction of phenylglyoxal with guanidino compounds under alkaline conditions, thereby causing lucigenin chemiluminescence. The chemiluminescent reaction of lucigenin in a mixture of phenylglyoxal and the guanidino compounds was applied to HPLC for guanidino compounds. The present chemiluminescence-HPLC system has a 2-fold greater sensitivity than chemiluminescence-HPLC using ninhydrin. Arginine, guanidine and methylguanidine were detected in serum from a hemodialysis patient with chronic renal failure.
Comparison of superoxide detection abilities of newly developed spin traps in the living cells.[Pubmed:19479584]
Free Radic Res. 2009 Jul;43(7):668-76.
This study compared the superoxide detection abilities of four spin traps, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide (DEPMPO), 5-(diphenylphosphinoyl)-5-methyl-1pyrroline N-oxide (DPPMPO) and 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) in living cells. Electron spin resonance (ESR) signals of the superoxide adducts were observed when spin traps were added to a suspension of human oral polymorphonuclear leukocytes (OPMNs) stimulated by phorbol 12-myristate 13-acetate. The ESR signal of the CYPMPO-superoxide adduct (CYPMPO-OOH) increased for 24 min after the initiation of the reaction, whereas the signals from DMPO-OOH and DPPMPO-OOH peaked at 6 and 10 min, respectively. The maximum concentrations of DMPO-OOH, DPPMPO-OOH and CYPMPO-OOH in OPMNs were 1.9, 6.0 and 10.7 microM, respectively. Furthermore, CYPMPO could more efficiently trap superoxide in blood PMNs compared with DEPMPO. From these results, it was concluded that CYPMPO performs better than DMPO, DPPMPO and DEPMPO for superoxide measurements in living cell systems because it has lower cytotoxicity and its superoxide adduct has a longer lifetime.
Analysis of hepatic oxidative stress status by electron spin resonance spectroscopy and imaging.[Pubmed:10802214]
Free Radic Biol Med. 2000 Mar 15;28(6):846-53.
Real-time detection of free radicals generated within the body may contribute to clarify the pathophysiological role of free radicals in disease processes. Of the techniques available for studying the generation of free radicals in biological systems, electron spin resonance (ESR) has emerged as a powerful tool for detection and identification. This article begins with a review of spin trapping detection of oxygen-centered radicals using X-band ESR spectroscopy and then describes the detection of superoxide and hydroxyl radicals by the spin trap 5,5-dimethyl-1-pyrroline-N-oxide and ESR spectroscopy in the perfusate from isolated perfused rat livers subjected to ischemia/reperfusion. This article also reviews the current status of ESR for the in vivo detection of free radicals and in vivo imaging of exogenously administered free radicals. Moreover, we show that in vivo ESR-computed tomography with 3-carbamoyl-2,2,5, 5-tetramethylpyrrolidine-1-oxyl may be useful for noninvasive anatomical imaging and also for imaging of hepatic oxidative stress in vivo.


