Cycloartenyl ferulateCAS# 21238-33-5 |
Quality Control & MSDS
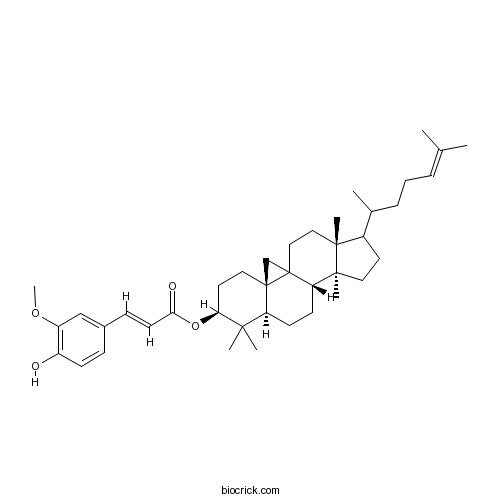
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21238-33-5 | SDF | Download SDF |
| PubChem ID | 134695320 | Appearance | Powder |
| Formula | C40H58O4 | M.Wt | 602.89 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(3R,6S,8R,11S,12S,16R)-7,7,12,16-tetramethyl-15-(6-methylhept-5-en-2-yl)-6-pentacyclo[9.7.0.01,3.03,8.012,16]octadecanyl] (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate | ||
| SMILES | CC(CCC=C(C)C)C1CCC2(C1(CCC34C2CCC5C3(C4)CCC(C5(C)C)OC(=O)C=CC6=CC(=C(C=C6)O)OC)C)C | ||
| Standard InChIKey | FODTZLFLDFKIQH-VVRSCMOVSA-N | ||
| Standard InChI | InChI=1S/C40H58O4/c1-26(2)10-9-11-27(3)29-18-20-38(7)33-16-15-32-36(4,5)34(19-21-39(32)25-40(33,39)23-22-37(29,38)6)44-35(42)17-13-28-12-14-30(41)31(24-28)43-8/h10,12-14,17,24,27,29,32-34,41H,9,11,15-16,18-23,25H2,1-8H3/b17-13+/t27?,29?,32-,33-,34-,37+,38-,39+,40?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cycloartenyl ferulate Dilution Calculator

Cycloartenyl ferulate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6587 mL | 8.2934 mL | 16.5868 mL | 33.1735 mL | 41.4669 mL |
| 5 mM | 0.3317 mL | 1.6587 mL | 3.3174 mL | 6.6347 mL | 8.2934 mL |
| 10 mM | 0.1659 mL | 0.8293 mL | 1.6587 mL | 3.3174 mL | 4.1467 mL |
| 50 mM | 0.0332 mL | 0.1659 mL | 0.3317 mL | 0.6635 mL | 0.8293 mL |
| 100 mM | 0.0166 mL | 0.0829 mL | 0.1659 mL | 0.3317 mL | 0.4147 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Regaloside C
Catalog No.:BCN9136
CAS No.:117591-85-2
- Regaloside F
Catalog No.:BCN9135
CAS No.:120601-65-2
- Eupalinolide H
Catalog No.:BCN9134
CAS No.:1402067-83-7
- Gingerglycolipid A
Catalog No.:BCN9133
CAS No.:145937-22-0
- 4'-O-Methylpyridoxine
Catalog No.:BCN9132
CAS No.:1464-33-1
- Tortoside A
Catalog No.:BCN9131
CAS No.:190655-16-4
- Trilinolein
Catalog No.:BCN9130
CAS No.:537-40-6
- Methyl 2-(methylamino)benzoate
Catalog No.:BCN9129
CAS No.:85-91-6
- Silybin
Catalog No.:BCN9128
CAS No.:802918-57-6
- γ-Asarone
Catalog No.:BCN9127
CAS No.:5353-15-1
- Bacopaside N1
Catalog No.:BCN9126
CAS No.:871706-74-0
- 3'-O-Methylbatatasin III
Catalog No.:BCN9125
CAS No.:101330-69-2
- Triglochinic acid
Catalog No.:BCN9138
CAS No.:31795-12-7
- Methoxyeugenol 4-O-rhamnosyl(1→2)glucoside
Catalog No.:BCN9139
CAS No.:903519-86-8
- Sinapoyl sinapaldehyde
Catalog No.:BCN9140
CAS No.:
- 6′′-O-β-D-Apiofuranosylapterin
Catalog No.:BCN9141
CAS No.:2188162-94-7
- Epirengynic acid
Catalog No.:BCN9142
CAS No.:1310146-00-9
- 6-(3-Methyl-2-oxobutyroyl)-7-methoxycoumarin
Catalog No.:BCN9143
CAS No.:2188162-96-9
- 3,4-Dihydroxybenzoyllupeol
Catalog No.:BCN9144
CAS No.:2231323-99-0
- Stephalonine L
Catalog No.:BCN9145
CAS No.:2379277-60-6
- Stephalonine N
Catalog No.:BCN9146
CAS No.:2376321-20-7
- Stephalonine P
Catalog No.:BCN9147
CAS No.:2376309-57-6
- cis-Nerolidol
Catalog No.:BCN9148
CAS No.:3790-78-1
- Stephalonine M
Catalog No.:BCN9149
CAS No.:2376321-05-8
Cycloartenyl Ferulate and beta-Sitosteryl Ferulate - Steryl Ferulates of gamma-Oryzanol - Suppress Intracellular Reactive Oxygen Species in Cell-based System.[Pubmed:31292340]
J Oleo Sci. 2019 Aug 1;68(8):765-768.
gamma-Oryzanol is a naturally occurring component of rice bran and consists of various steryl ferulates. The antioxidant activities of gamma-oryzanol have mostly been demonstrated in cell-free systems. Therefore, we determined whether steryl ferulate of gamma-oryzanol suppress spontaneous intracellular reactive oxygen species (ROS) in cell-based systems. We found that Cycloartenyl ferulate and beta-sitosteryl ferulate suppressed spontaneous intracellular ROS in a similar way to N-acetylcysteine and alpha-tocopherol.
A rapid and eco-friendly method for determination of the main components of gamma-oryzanol in equestrian dietary and nutritional supplements by liquid chromatography-Tandem mass spectrometry.[Pubmed:31085396]
J Pharm Biomed Anal. 2019 Aug 5;172:339-348.
Gamma-oryzanol (GO) has gained special attention in the equine sports industry in recent years due to its touted properties, including the fact that it may cause anabolic effects on muscle growth and reduce fatigue. Many manufactures offer supplements containing GO as a naturally occurring anabolic substance; however, some producers do not declare its presence in product compositions. Taking into consideration the touted properties of GO, its ambiguous effectiveness and the open character of the Prohibited Substances List established by the Federation Equestre Internationale, there is an urgent need to elaborate procedures for the estimation of horse exposure to GO during supplementation, as well as during routine analysis of supplements. This work describes the development and validation of the method for determination of the four main GO components, i.e., Cycloartenyl ferulate, 24-methylenecycloartanyl ferulate, campesteryl ferulate and beta-sitosteryl ferulate, in equestrian supplements based on LC-MS/MS after a simple ultrasound-assisted extraction (Eco-Scale score value of 76). The analytical performance achieved satisfactory results in terms of linearity (R(2) > 0.9910), sensitivity (LODs ranged from 0.4 to 1.9 ng/mL), intra- and interday accuracy (from 90.4-115.8%), precision (CV < 9.6%) and recovery (from 87.6-108.6%) for all of the investigated compounds. The method was successfully applied to the analysis of thirty equestrian supplements.
Pharmacokinetics of vitamin E, gamma-oryzanol, and ferulic acid in healthy humans after the ingestion of a rice bran-enriched porridge prepared with water or with milk.[Pubmed:29978378]
Eur J Nutr. 2019 Aug;58(5):2099-2110.
PURPOSE: In this study, we investigated the absorption and excretion kinetics of antioxidant dietary phytochemicals (vitamin E, gamma-oryzanol, and ferulic acid) in healthy humans after the ingestion of an oatmeal porridge supplemented with rice bran extract (RBE) prepared with water or with whole milk, and we compared it with the intake of an equivalent dose of the rice bran content, in the form of RBE oil. METHODS: Twelve healthy volunteers (6 men and 6 women) orally ingested RBE oil (2 g) or RBE-enriched porridge (35 g, including 2-g RBE) with water or with milk, in a three-armed, crossover trial. Blood and urine samples were collected at baseline and up to 24 h after intake. Vitamin E (alpha-, beta-, gamma-, and delta-tocopherols and tocotrienols), ferulic acid (FA), and gamma-oryzanol (ORY) were quantified by HPLC. RESULTS: The ingestion of RBE-fortified oatmeal porridge and RBE oil significantly increased FA concentrations in plasma, showing faster absorption and higher maximum plasma concentrations after the intake of the porridges, irrespective of the addition of water or milk. At least part of the FA could have been hydrolyzed from ORY. However, plasma vitamin E concentrations did not increase from baseline, and no intact FA esters (Cycloartenyl ferulate, 24-methylenecycloartanyl ferulate, campesteryl ferulate, and beta-sitosteryl ferulate) were detected in plasma or urine with any of the meal treatments. CONCLUSIONS: Rice bran extract-enriched porridge and, to a lesser extent, RBE oil, provide relevant sources of bioaccessible and bioavailable ferulic acid, and could be further developed into functional foods with health potential.
Presence of orally administered rice bran oil gamma-oryzanol in its intact form in mouse plasma.[Pubmed:27878202]
Food Funct. 2016 Dec 7;7(12):4816-4822.
Although the beneficial effects (e.g., lipid-lowering activity) of gamma-oryzanol (OZ), a mixture of ferulic acid esters of plant sterols and triterpene alcohols, have been extensively investigated, few studies have evaluated the absorption and metabolism of OZ. Moreover, it is unclear whether OZ, once ingested, is directly absorbed by the intestine into the bloodstream at a sufficient level to exhibit activity. Here, we prepared OZ concentrate from purified rice bran oil (Rice Oil OZ), determined the concentration of OZ in the preparation (Cycloartenyl ferulate equivalent concentration; 52.2%), and then carried out chromatography-mass spectrometry analysis of plasma samples from mice after oral administration of Rice Oil OZ. The OZ concentrations of plasma from the control (vehicle-treated) mice were low (trace levels); however, at 5 h after a single oral administration of the Rice Oil OZ (600 mg per kg body weight), the levels significantly increased, reaching 17.6 ng mL(-1) for Cycloartenyl ferulate, 28.2 ng mL(-1) for 24-methylenecycloartanyl ferulate isomers, 15.6 ng mL(-1) for campesteryl ferulate, and 5.1 ng mL(-1) for beta-sitosteryl ferulate, respectively, expressed in equivalence of Cycloartenyl ferulate in plasma. These results provided the first mass spectrometric evidence suggesting that a portion of orally administered OZ is directly absorbed by the intestine and is present in the intact form in plasma. The presence of a significant amount of OZ in its intact form in plasma may explain the beneficial effects of OZ in vivo.
Cycloartenyl Ferulate Inhibits Paraquat-Induced Apoptosis in HK-2 Cells With the Involvement of ABCC1.[Pubmed:26358524]
J Cell Biochem. 2016 Apr;117(4):872-80.
Nephrotoxicity induced by chemicals such as paraquat (PQ) is a common clinical phenomenon; therefore, searching for drugs with renal protective effect is of a great practical significance. Our previous investigation found that Cycloartenyl ferulate (CF) can antagonize the cytotoxic effect of PQ, and recent studies also revealed a variety of bioactivities of CF. However, specific molecular mechanisms underlying the protective effect of CF have not been explored yet. HPLC detection of PQ content indicated that CF reduced PQ accumulation in HK-2 cells and thereby improved cell survival. Western blot results showed that both PQ and CF did not affect the expression of ABCB1; however, while PQ suppressed the expression of ABCC1, CF upregulated ABCC1 expression and thereby reversed the inhibitory effect of PQ on ABCC1 expression. Meanwhile, HK-2 cells did not express ABCG2. When the expression of ABCC1 was knocked down with siRNA, the inhibitory effect of CF on intracellular PQ accumulation was blocked. Further flow cytometric analysis showed that while PQ significantly induced the appearance of sub-G1 apoptotic peak in cells, CF evidently inhibited apoptosis. TUNEL-DAPI double-staining also detected that PQ significantly induced the occurrence of DNA fragmentation in cells, whereas CF effectively inhibited the effect of PQ. Further results showed that ABCC1 siRNA effectively abolished the protective effect of CF on PQ-induced apoptosis. Taken together, these data demonstrated that in HK-2 cells, CF could antagonize PQ-induced toxicity with the involvement of regulatiion of ABCC1 protein expression, which provides a new strategy for treatments of nephrotoxicity.
Simultaneous HPLC quantification of five major triterpene alcohol and sterol ferulates in rice bran oil using a single reference standard.[Pubmed:24262565]
Food Chem. 2014 Apr 1;148:329-34.
A high performance liquid chromatography (HPLC) method was developed for simultaneous quantification of five major triterpene alcohol and sterol ferulates in rice bran oils (RBO) with a single internal standard, Cycloartenyl ferulate. The five compounds are Cycloartenyl ferulate (1), 24-methylene cycloartanyl ferulate (2), campesteryl ferulate (3), sitosteryl ferulate (4) and stigmastanyl ferulate (5). All five compounds had good linear concentration-measurement relationships (r(2) >/= 0.9995) and possessed similar relative response factors. The relative deviation of this method was less than 2.5% for intra- and inter-day assays, and the average recovery varied from 95.1% to 99.4%. The new method was validated by comparing the amount of 24-methylene cycloartanyl ferulate (2) in 17 RBO samples obtained with this method and that with an external standard method. This method was also successfully applied to determine five major triterpene alcohol and sterol ferulates in 17 batches of RBO samples. The results demonstrated that the present method could be utilised for quality control of RBO since some of the reference standards are not commercially available.
Isolation and identification of phenolic compounds accumulated in brown rice grains ripened under high air temperature.[Pubmed:24168351]
J Agric Food Chem. 2013 Dec 11;61(49):11921-8.
This study aimed to examine the compounds increasing or decreasing in concentration in brown rice grains ripened under high air temperature during ripening using a heat-tolerant cultivar Fusaotome, a heat-intolerant cultivar Hatsuboshi, and an intermediate cultivar Koshihikari. 6-O-Feruloylsucrose (1), 3',6-di-O-sinapoylsucrose (2), 3'-O-sinapoyl-6-O-feruloylsucrose (3), 3',6-di-O-feruloylsucrose (4), Cycloartenyl ferulate (5), and 24-methylenecycloartanyl ferulate (6) were isolated from the extracts of brown rice grains. The structures of the isolated compounds (1-6) were elucidated on the basis of spectroscopic analyses. The mean concentrations of compounds 2, 3, and 6 in the grains ripened under high air temperature were markedly higher than those ripened under normal air temperature. In contrast, the mean concentration of compound 5 in the grains ripened under high air temperature was markedly lower than those ripened under normal air temperature. Thus, compounds 2, 3, 5, and 6 constitute potential biomarkers of heat stress in the cultivars used. The mean concentrations of compound 4 in the grains of Fusaotome were the highest in all cultivars. In contrast, the mean concentration of compound 5 in the grains of Fusaotome was the lowest. Therefore, the unique composition of heat-tolerant Fusaotome combines a high concentration of compound 4 with a low concentration of compound 5.
The reversal of paraquat-induced mitochondria-mediated apoptosis by cycloartenyl ferulate, the important role of Nrf2 pathway.[Pubmed:23954820]
Exp Cell Res. 2013 Nov 1;319(18):2845-55.
In this study, we demonstrate the protective effects of Cycloartenyl ferulate (CF) against Paraquat (PQ)-induced cytotoxicity and elucidate the underlying molecular mechanisms. The results show that, CF could reverse the PQ-induced growth inhibition and release of lactate dehydrogenase in HK-2 human proximal tubular cells. Treatment with PQ induced apoptosis in HK-2 cells, as evidenced by accumulation of sub-G1 cell population, chromatin condensation, DNA fragmentation, and translocation of phosphatidylserine, which were significantly attenuated by co-incubation with CF. Mitochondria-mediated apoptosis pathway contributed importantly to PQ-induced apoptosis, as revealed by the activation of caspase-3/-9, cleavage of PARP, depletion of mitochondrial membrane potential regulated by Bcl-2 family members, and overproduction of reactive oxygen species, which were also effectively blocked by CF. Moreover, treatments of PQ strongly inhibited the expression of Nrf2 and the downstream effectors, HO1 and NQO1. However, co-treatment with CF effectively reversed this action of PQ. Furthermore, silencing of Nrf2 by the siRNA technique significantly blocked the cytoprotective effects of CF against PQ-induced apoptosis, which suggest the important role of Nrf2 signaling pathway an cell apoptosis induced by PQ. Taken together, this study provides a novel strategy for molecular intervention against PQ-induced nephrotoxicity by using phytochemicals.
Evaluation of gamma-oryzanol content and composition from the grains of pigmented rice-germplasms by LC-DAD-ESI/MS.[Pubmed:23587158]
BMC Res Notes. 2013 Apr 15;6:149.
BACKGROUND: Rice is the staple food and one of the world's three major grain crops. Rice contains more than 100 bioactive substances including phytic acid, isovitexin, gamma-oryzanol, phytosterols, octacosanol, squalene, gamma-aminobutyric acid (GABA), tocopherol, tocotrienol derivatives, etc. Out of them, gamma-oryzanol is known to have important biological profile such as anti-oxidants, inhibitor of cholesterol oxidation, reduce serum cholesterol levels in animals, effective in the treatment of inflammatory diseases, inhibit tumor growth, reduce blood pressure and promotes food storage stability when used as a food additive, etc. Hence in the present investigation, we aimed to evaluate the content and composition of gamma-oryzanol from pigmented rice germplasms using a liquid chromatography with diode array detection and electrospray ionization-mass spectrometry (LC-DAD-ESI/MS). FINDINGS: In the present study, 33 exotic pigmented rice accessions (red, white and purple) have been evaluated. Among them, the contents of gamma-oryzanol varied from 3.5 to 21.0 mg/100 g with a mean of 11.2 mg/100 g. A total of ten components of gamma-oryzanol including Delta(7)-stigmastenyl ferulate were identified of which, Cycloartenyl ferulate, 24-methylenecycloartanyl ferulate, campesteryl ferulate and sitosteryl ferulate were identified as the major components. The mean proportions of steryl ferulates were in the descending order of 24-methylenecycloartanyl ferulate > Cycloartenyl ferulate > campesteryl ferulate > sitosteryl ferulate > Delta(7)-campestenyl ferulate > campestanyl ferulate > sitostanyl ferulate > Delta(7)-stigmastenyl ferulate > stigamsteryl ferulate > Delta(7)-sitostenyl ferulate. Almost 11 accessions (33%) showed higher content than the control rice Chucheongbyeo and higher proportions ranged from 10 to 15 mg/100 g. Interestingly, the red rice accession Liberian Coll. B11/B-11 (21.0 mg/100 g) showed higher content gamma-oryzanol than control rice Jeokjinjubyeo (19.1 mg/100 g) and the purple rice accession Padi Adong Dumarat, Mardi No.4376 (20.3 mg/100 g) showed a similar content with control rice Heugjinjubyeo (21.4 mg/100 g). CONCLUSIONS: Most of analyzed rice accessions were found to possess higher contents of gamma-oryzanol than the control rice, Chucheongbyeo. In particular, the red accessions showed highest content than the white and purpleaccessions. The content and composition of gamma-oryzanol in 33 exotic pigmented rice accessions have been evaluated and compared significantly by the present investigation.
Preparative separation of triterpene alcohol ferulates from rice bran oil using a high performance counter-current chromatography.[Pubmed:23561190]
Food Chem. 2013 Aug 15;139(1-4):919-24.
A novel method for the separation of two major triterpene alcohol ferulates from rice bran oil (RBO) was developed using a high performance counter-current chromatography (HPCCC). A two-phase solvent system of n-hexane-acetonitrile (1:1, v/v) was applied to purify Cycloartenyl ferulate (CAF) and 24-methylene cycloartanyl ferulate (24-mCAF) from RBO. The yields were 20.50+/-2.60 mg CAF and 12.62+/-1.15 mg 24-mCAF from 390 mg RBO through a two-step separation procedure. The purities of the two compounds were 97.97+/-0.90% and 95.50+/-0.75%, respectively, as determined by high performance liquid chromatography (HPLC). Their chemical structures were confirmed by ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS), and (1)H, (13)C and 2D nuclear magnetic resonance (NMR). This represents the first report on direct separation of CAF and 24-mCAF from RBO by HPCCC.


