Cyclo(Tyr-Pro)CAS# 5654-84-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5654-84-2 | SDF | Download SDF |
| PubChem ID | 371682 | Appearance | Powder |
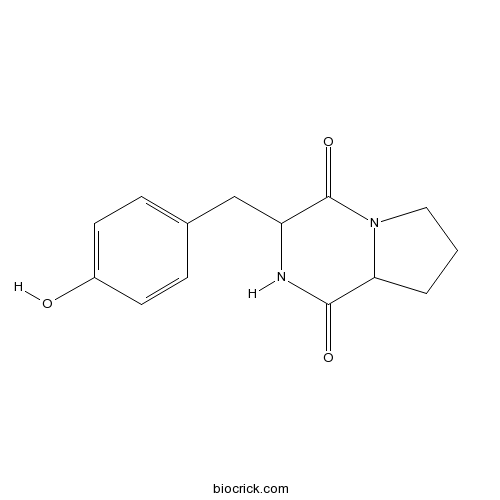
| Formula | C14H16N2O3 | M.Wt | 260.29 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-[(4-hydroxyphenyl)methyl]-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| SMILES | C1CC2C(=O)NC(C(=O)N2C1)CC3=CC=C(C=C3)O | ||
| Standard InChIKey | LSGOTAXPWMCUCK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H16N2O3/c17-10-5-3-9(4-6-10)8-11-14(19)16-7-1-2-12(16)13(18)15-11/h3-6,11-12,17H,1-2,7-8H2,(H,15,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cyclo(Tyr-Pro) shows antibacterial activity towards several marine bacterial species, it also shows weak antagonistic activity against VEGFR2 -CD. Cyclo(Tyr-Pro) and cyclo(Pro-Val) are toxic to both suspension cells and seedlings of Pinus thunbergii, which may offer some clues to research the mechanism of pine wilt disease caused by pine wood nematode. |
| Targets | VEGFR | Antifection |
| In vitro | Antimicrobial activity of liposome encapsulated cyclo(L-tyrosyl-L-prolyl)[Reference: WebLink]Die Pharmazie - An International Journal of Pharmaceutical Sciences,2011,66(6):421-3.Various studies have shown the potentially beneficial biological activities of cyclic dipeptides and in particular, cyclo(L-tyrosyl-L-prolyl) (Cyclo(Tyr-Pro)) has shown fair antibacterial activity in vitro. This study aimed to determine if liposome encapsulation would have any significant effects on the antibacterial activity of this compound. Two Cyclic Dipeptides from Pseudomonas fluorescens GcM5-1A Carried by the Pine Wood Nematode and Their Toxicities to Japanese Black Pine Suspension Cells and Seedlings in vitro.[Pubmed: 19259494 ]J Nematol. 2007 Sep;39(3):243-7.
Antibacterial Metabolites from Marine Bacterium Pseudomonas sp.[Reference: WebLink]Natural Product Research & Development, 2009,21(3): 420-3.
|
| Kinase Assay | Discovery on antagonists of VEGFR2-CD produced by Streptomyces strain I06A-02832.[Reference: WebLink]Chinese Journal of Antibiotics,2014,6:408-12,421.To discover antagonists of VEGFR2-CD from the fermentation broth produced by streptomyces strain I06A-02832. |
| Structure Identification | Journal of Crystallographic and Spectroscopic Research December 1992,22(6):643-649.Cyclodipeptides: Structure and conformation of cyclo(tyrosyl-prolyl)[Reference: WebLink]
|

Cyclo(Tyr-Pro) Dilution Calculator

Cyclo(Tyr-Pro) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8419 mL | 19.2093 mL | 38.4187 mL | 76.8374 mL | 96.0467 mL |
| 5 mM | 0.7684 mL | 3.8419 mL | 7.6837 mL | 15.3675 mL | 19.2093 mL |
| 10 mM | 0.3842 mL | 1.9209 mL | 3.8419 mL | 7.6837 mL | 9.6047 mL |
| 50 mM | 0.0768 mL | 0.3842 mL | 0.7684 mL | 1.5367 mL | 1.9209 mL |
| 100 mM | 0.0384 mL | 0.1921 mL | 0.3842 mL | 0.7684 mL | 0.9605 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Hydroxy-3,4-dimethoxybenzoic acid
Catalog No.:BCN6535
CAS No.:5653-46-3
- Angelic acid
Catalog No.:BCN3410
CAS No.:565-63-9
- Steppogenin
Catalog No.:BCN5760
CAS No.:56486-94-3
- 4,9-Dihydroxy-alpha-lapachone
Catalog No.:BCN5758
CAS No.:56473-67-7
- Eichlerianic acid
Catalog No.:BCN5757
CAS No.:56421-13-7
- Methyl eichlerianate
Catalog No.:BCN5756
CAS No.:56421-12-6
- Tetrahydrolachnophyllum lactone
Catalog No.:BCN4759
CAS No.:56407-87-5
- Hinokiol
Catalog No.:BCN5759
CAS No.:564-73-8
- 11α-Hydroxyandrost-4-ene-3,17-dione
Catalog No.:BCC8432
CAS No.:564-33-0
- Doxycycline
Catalog No.:BCN2397
CAS No.:564-25-0
- Sclareolide
Catalog No.:BCC6492
CAS No.:564-20-5
- Hop-17(21)-en-3-ol
Catalog No.:BCN5755
CAS No.:564-14-7
- Cyclo(Pro-Leu)
Catalog No.:BCN2426
CAS No.:5654-86-4
- Cyclo(Pro-Val)
Catalog No.:BCN2420
CAS No.:5654-87-5
- (-)-Bornyl acetate
Catalog No.:BCN2636
CAS No.:5655-61-8
- PIT
Catalog No.:BCC7151
CAS No.:56583-49-4
- 3-Hydroxy-2-phenyl-propanamide
Catalog No.:BCN3905
CAS No.:56598-62-0
- 7-Keto-dehydroepiandrosterone
Catalog No.:BCC8780
CAS No.:566-19-8
- 7Beta-Hydroxycholesterol
Catalog No.:BCN2751
CAS No.:566-27-8
- Formestane
Catalog No.:BCC4369
CAS No.:566-48-3
- BOP reagent
Catalog No.:BCC2807
CAS No.:56602-33-6
- H-D-Phe(4-NO2)-OH
Catalog No.:BCC3274
CAS No.:56613-61-7
- D-Phenylglycinol
Catalog No.:BCC2712
CAS No.:56613-80-0
- Z-Arg-OH.HCl
Catalog No.:BCC3061
CAS No.:56672-63-0
Two Cyclic Dipeptides from Pseudomonas fluorescens GcM5-1A Carried by the Pine Wood Nematode and Their Toxicities to Japanese Black Pine Suspension Cells and Seedlings in vitro.[Pubmed:19259494]
J Nematol. 2007 Sep;39(3):243-7.
Pseudomonas fluorescens GcM5-1A, isolated from the pine wood nematode (PWN), Bursaphelenchus xylophilus, was cultured in Luria Broth medium (LB). The clarified culture was extracted with ethyl acetate, and two dipeptides were purified from the extract. The chemical structures of 1 and 2 were identified as cyclo(-Pro-Val-)and cyclo(-Pro-Tyr-), respectively, by MS, (1)H NMR, (13)C NMR,(1)H-(1)H COSY, 1H -(13)C COSY spectra. Bioassay results showed that the two compounds were toxic to both suspension cells and seedlings of Pinus thunbergii, which may offer some clues to research the mechanism of pine wilt disease caused by PWN.


