BOP reagentPeptide coupling reagent CAS# 56602-33-6 |
- MK-5172 hydrate
Catalog No.:BCC1763
CAS No.:1350462-55-3
- Telaprevir (VX-950)
Catalog No.:BCC2107
CAS No.:402957-28-2
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Danoprevir (RG7227)
Catalog No.:BCC2106
CAS No.:850876-88-9
- Narlaprevir
Catalog No.:BCC1785
CAS No.:865466-24-6
- Simeprevir
Catalog No.:BCC1949
CAS No.:923604-59-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

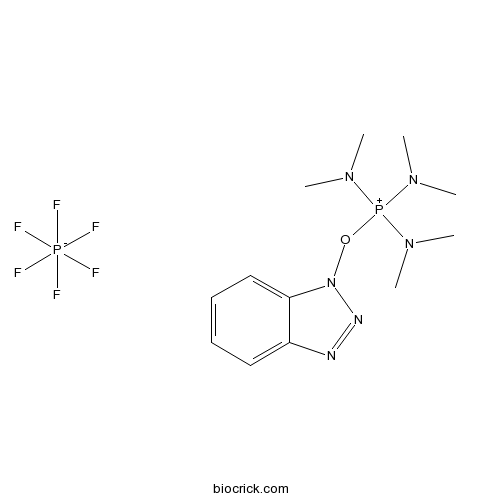
| Cas No. | 56602-33-6 | SDF | Download SDF |
| PubChem ID | 151348 | Appearance | Powder |
| Formula | C12H22F6N6OP2 | M.Wt | 442.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >4.43mg/ml in EtOH | ||
| Chemical Name | benzotriazol-1-yloxy-tris(dimethylamino)phosphanium;hexafluorophosphate | ||
| SMILES | CN(C)[P+](N(C)C)(N(C)C)ON1C2=CC=CC=C2N=N1.F[P-](F)(F)(F)(F)F | ||
| Standard InChIKey | MGEVGECQZUIPSV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H22N6OP.F6P/c1-15(2)20(16(3)4,17(5)6)19-18-12-10-8-7-9-11(12)13-14-18;1-7(2,3,4,5)6/h7-10H,1-6H3;/q+1;-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BOP reagent is a coupling reagent for peptide synthesis. |

BOP reagent Dilution Calculator

BOP reagent Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2599 mL | 11.2994 mL | 22.5989 mL | 45.1977 mL | 56.4972 mL |
| 5 mM | 0.452 mL | 2.2599 mL | 4.5198 mL | 9.0395 mL | 11.2994 mL |
| 10 mM | 0.226 mL | 1.1299 mL | 2.2599 mL | 4.5198 mL | 5.6497 mL |
| 50 mM | 0.0452 mL | 0.226 mL | 0.452 mL | 0.904 mL | 1.1299 mL |
| 100 mM | 0.0226 mL | 0.113 mL | 0.226 mL | 0.452 mL | 0.565 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A peptide coupling reagent. Can be used in the preparation of phenyl esters of amino acids which have been shown to be valuable as blocked derivatives of amino acids in the field of peptide synthesis.
- Formestane
Catalog No.:BCC4369
CAS No.:566-48-3
- 7Beta-Hydroxycholesterol
Catalog No.:BCN2751
CAS No.:566-27-8
- 7-Keto-dehydroepiandrosterone
Catalog No.:BCC8780
CAS No.:566-19-8
- 3-Hydroxy-2-phenyl-propanamide
Catalog No.:BCN3905
CAS No.:56598-62-0
- PIT
Catalog No.:BCC7151
CAS No.:56583-49-4
- (-)-Bornyl acetate
Catalog No.:BCN2636
CAS No.:5655-61-8
- Cyclo(Pro-Val)
Catalog No.:BCN2420
CAS No.:5654-87-5
- Cyclo(Pro-Leu)
Catalog No.:BCN2426
CAS No.:5654-86-4
- Cyclo(Tyr-Pro)
Catalog No.:BCN2421
CAS No.:5654-84-2
- 2-Hydroxy-3,4-dimethoxybenzoic acid
Catalog No.:BCN6535
CAS No.:5653-46-3
- Angelic acid
Catalog No.:BCN3410
CAS No.:565-63-9
- Steppogenin
Catalog No.:BCN5760
CAS No.:56486-94-3
- H-D-Phe(4-NO2)-OH
Catalog No.:BCC3274
CAS No.:56613-61-7
- D-Phenylglycinol
Catalog No.:BCC2712
CAS No.:56613-80-0
- Z-Arg-OH.HCl
Catalog No.:BCC3061
CAS No.:56672-63-0
- Batatasin III
Catalog No.:BCN3596
CAS No.:56684-87-8
- 2-Benzylacrylic acid
Catalog No.:BCC8564
CAS No.:5669-19-2
- Orteronel
Catalog No.:BCC1823
CAS No.:566939-85-3
- (+/-)-Vestitol
Catalog No.:BCN6814
CAS No.:56701-24-7
- Z-Glu-OMe
Catalog No.:BCC2779
CAS No.:5672-83-3
- Icariside I
Catalog No.:BCN3463
CAS No.:56725-99-6
- 1-Methoxyallocryptopine
Catalog No.:BCN7454
CAS No.:56743-52-3
- Phenylalanine betaine
Catalog No.:BCN5761
CAS No.:56755-22-7
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
Applications of BOP reagent in solid phase synthesis. Advantages of BOP reagent for difficult couplings exemplified by a synthesis of [Ala 15]-GRF(1-29)-NH2.[Pubmed:2896637]
Int J Pept Protein Res. 1988 Jan;31(1):86-97.
The BOP reagent [benzotriazol-l-yl-oxy-tris-(dimethylamino)phosphonium hexa-fluorophosphate] introduced by Castro et al. [Tetrahedron Lett. (1975) 14, 1219-1222] is ideally suited for solid phase peptide synthesis. The rate of coupling using BOP compared favorably to DCC and other methods of activation including the symmetrical anhydride and DCC/HOBt procedures. BOP couplings using the solid phase procedure proceeded more rapidly and to a greater degree of completion for peptide bond formations that were previously determined to be very slow using the conventional DCC method. Stepwise solid phase peptide synthesis using BOP was successfully utilized for the preparation of the (22-29) and (13-29) fragments of [Ala15]-GRF(1-29)-NH2. Single couplings with 3 equiv. BOP and Boc-amino acids and 5.3 equiv. of diisopropylethylamine in DMF were used for each cycle. The yields of the fragments were superior and the purities comparable using the BOP procedure (single couplings) to those observed using multiple couplings via the DCC coupling method. A total synthesis of [Ala15]-GRF(1-29)-NH2 was also carried out using the BOP procedure (single couplings and 3 equiv. BOP and Boc-amino acids and 5.3 equiv. diisopropylethylamine in DMF for each cycle). Multiple couplings were only required for Boc-Asn-OH due to the proposed formation of Boc-aminosuccinimide during activation. The resultant GRF(1-29) analog was comparable to a control prepared with multiple DCC couplings under optimized conditions. In a parallel study, unprotected Boc-(hydroxy)-amino acids were successfully coupled with the BOP reagent. However, the number of coupling cycles after the introduction of unprotected hydroxy-amino acid must be minimal (less than 10). The use of the BOP reagent with unprotected Tyr in solid phase peptide synthesis was also clearly established.
BOP reagent for the coupling of pGlu and Boc-His(Tos) in solid phase peptide synthesis.[Pubmed:2323890]
Int J Pept Protein Res. 1990 Feb;35(2):89-94.
The model peptide TRH was successfully synthesized using benzotriazol-1-yl-oxy-tris(dimethylamino)phosphonium hexafluorophosphate (BOP reagent). The coupling reactions were carried out in N,N-dimethylformamide or N-methylpyrrolidone. These solvents allowed the incorporation of the N-terminal pyroglutamic acid residue into the peptide chain, without using the derivative bearing the N-benzyloxycarbonyl group, which acts as a solubility promoter. A comparative racemization study showed that Boc-His(Tos) can be coupled by means of BOP reagent with less racemization than with DCC when the amount of diisopropylethylamine (DIEA) is kept minimal (same ratio of equivalents as for Boc-His(Tos), i.e. 3 equiv.). However, with the use of a larger amount of DIEA in the coupling mixture (9 equiv.), approximately 3% of epimer was found in the crude product. Our study showed that even under low DIEA conditions, the rate of coupling of the residues with BOP remained comparable to that observed with DCC.
Total chemical synthesis of ubiquitin using BOP reagent: biochemical and immunochemical properties of the purified synthetic product.[Pubmed:2562486]
Pept Res. 1989 Nov-Dec;2(6):381-8.
The complete ubiquitin molecule (76 residues) has been synthesized by the solid-phase method of Merrifield by using BOP as the coupling reagent. The crude product was purified by gel filtration, middle-pressure liquid chromatography and ion-exchange chromatography. Seven mg of a ca. 95% pure peptide was finally obtained; the overall yield of the synthesis was 1%. The final product was controlled by amino acid analysis and sequencing, HPLC and FAB-Mass spectrometry. Synthetic ubiquitin was found to be antigenically active in immunoblotting experiments and in an enzyme-linked immunosorbent assay with anti-ubiquitin antibodies as well as with anti-ubiquitin autoantibodies from autoimmune patients. Its activity was controlled in a conjugation enzymatic system and was found similar to that of commercial ubiquitin. The successful total synthesis of ubiquitin opens the way to the preparation of various stable analogs that should be useful for studying the intracellular metabolism of this molecular and its involvement in the protein degradation pathway.


