CycleanineCAS# 518-94-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 518-94-5 | SDF | Download SDF |
| PubChem ID | 121313 | Appearance | White crystalline powder |
| Formula | C38H42N2O6 | M.Wt | 622.75 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
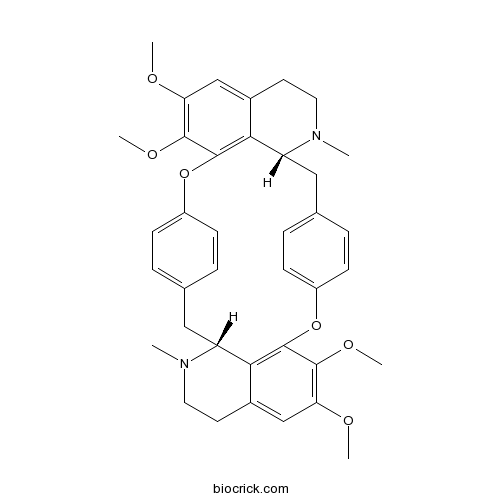
| SMILES | CN1CCC2=CC(=C(C3=C2C1CC4=CC=C(C=C4)OC5=C6C(CC7=CC=C(O3)C=C7)N(CCC6=CC(=C5OC)OC)C)OC)OC | ||
| Standard InChIKey | ANOXEUSGZWSCQL-LOYHVIPDSA-N | ||
| Standard InChI | InChI=1S/C38H42N2O6/c1-39-17-15-25-21-31(41-3)35(43-5)37-33(25)29(39)19-23-7-11-28(12-8-23)46-38-34-26(22-32(42-4)36(38)44-6)16-18-40(2)30(34)20-24-9-13-27(45-37)14-10-24/h7-14,21-22,29-30H,15-20H2,1-6H3/t29-,30-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | The biological screening of cycleanine and the root bark alkaloidal extract revealed potent antibacterial, antifungal, antiplasmodial, and cytotoxic activities. Cycleanine, like its isomer – tetrandrine isolated from T. subcordata, could be a potential new anti-ovarian cancer agent acting through the apoptosis pathway. It also shows antiplasmodial activities against Plasmodium falciparum 3D7 with IC50 values of 0.08 µM.Cycleanine may have anti-inflammatory activity. Cycleanine markedly inhibited Na(+),K(+)-ATPase activity with an IC(50) value of 6.2 x 10(-4)M. It slightly inhibited Mg(2+)-ATPase, H(+)-ATPase, and Ca(2+)-ATPase, it might interact with the enzyme in Na.E(1)-P form and prevents the reaction step from Na.E(1)-P to E(2)-P. |
| Targets | ATPase | Sodium Channel | Potassium channel | Calcium Channel | NO | Antifection |
| In vitro | Cytotoxicity effects and apoptosis induction by cycleanine and tetrandrine.[Reference: WebLink]Planta Medica, 2016, 81(S 01):S1-S381.Ovarian cancer remains one of the main causes of death in all gynecologic malignancies [1]. Natural products continue to be important sources of clinically approved anti-cancer drugs [2, 3]. Triclisia subcordata Oliv (Menispermeaceae) is a medicinal plant traditionally used for the treatment of various diseases [4], including cancer, in West Africa. This study aims to evaluate the in vitro anti-ovarian cancer activities of the crude extracts and the isolated components in T. subcordata.
Anti-malarial Activity of Isoquinoline Alkaloids from the Stem Bark of Actinodaphne macrophylla.[Pubmed: 26594753]Nat Prod Commun. 2015 Sep;10(9):1541-2.
Antibacterial, antifungal, antiplasmodial, and cytotoxic activities of Albertisia villos[Pubmed: 15234773 ]J Ethnopharmacol. 2004 Aug;93(2-3):331-5.Albertisia villosa (Menispermaceae) is a subtropical medicinal plant that is widely used in traditional African medicines against various diseases. |
| Kinase Assay | Inhibitory effect of bisbenzylisoquinoline alkaloids on nitric oxide production in activated macrophages.[Pubmed: 7505581]Inhibition of Na+,K+-ATPase by the extract of Stephania cephararantha Hayata and bisbenzylisoquinoline alkaloid cycleanine, a major constituent.[Pubmed: 12907236 ]Biochemical Pharmacology, 2003, 66(3):379-385.The Stephania cephararantha HAYATA extract, and its constituent bisbenzylisoquinoline alkaloids, such as Cycleanine, cepharanthine, isotetrandrine, berbamine, homoaromoline, and cepharanoline were studied for effects on Na(+),K(+)-ATPase activity. Biochem Pharmacol. 1993 Dec 3;46(11):1887-92.Bisbenzylisoquinoline (BBI) alkaloids are anti-inflammatory constituents of plants of the families Menispermaceae and Ranunculaceae, which have been used as folk remedies in Japan and China. |
| Cell Research | Synthesis of (aminoalkyl)cycleanine analogues: cytotoxicity, cellular uptake, and apoptosis induction in ovarian cancer cells.[Pubmed: 29588214]Bioorg Med Chem Lett. 2018 May 15;28(9):1652-1656.Our previous studies demonstrated that Cycleanine, a macrocyclic bisbenzylisoquinoline (BBIQ) alkaloid, showed potent anti-ovarian cancer activity via apoptosis induction.
|
| Structure Identification | Phytochemistry, 1980, 19(8):1837-1840.Cycleanine from Synclisia scabrida: conformational information from the 1H NMR spectrum at 300 MHz.[Reference: WebLink]
|

Cycleanine Dilution Calculator

Cycleanine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6058 mL | 8.0289 mL | 16.0578 mL | 32.1156 mL | 40.1445 mL |
| 5 mM | 0.3212 mL | 1.6058 mL | 3.2116 mL | 6.4231 mL | 8.0289 mL |
| 10 mM | 0.1606 mL | 0.8029 mL | 1.6058 mL | 3.2116 mL | 4.0145 mL |
| 50 mM | 0.0321 mL | 0.1606 mL | 0.3212 mL | 0.6423 mL | 0.8029 mL |
| 100 mM | 0.0161 mL | 0.0803 mL | 0.1606 mL | 0.3212 mL | 0.4014 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Xanthopurpurin
Catalog No.:BCN6723
CAS No.:518-83-2
- Emodin
Catalog No.:BCN5649
CAS No.:518-82-1
- Corydaline
Catalog No.:BCN2342
CAS No.:518-69-4
- Tetrandrine
Catalog No.:BCN5955
CAS No.:518-34-3
- (-)-beta-Peltatin
Catalog No.:BCN3606
CAS No.:518-29-6
- Podophyllotoxin
Catalog No.:BCN5957
CAS No.:518-28-5
- Evodiamine
Catalog No.:BCN1092
CAS No.:518-17-2
- Dehydroglyasperin D
Catalog No.:BCN6829
CAS No.:517885-72-2
- Rengynic acid
Catalog No.:BCN5644
CAS No.:517883-38-4
- Carteolol HCl
Catalog No.:BCC6466
CAS No.:51781-21-6
- Mefloquine hydrochloride
Catalog No.:BCC1737
CAS No.:51773-92-3
- Valechlorine
Catalog No.:BCN2763
CAS No.:51771-49-4
- Isomaculosidine
Catalog No.:BCN7069
CAS No.:518-96-7
- 3,3'-Di-O-methylellagic acid 4'-glucoside
Catalog No.:BCN1431
CAS No.:51803-68-0
- Nimesulide
Catalog No.:BCC4435
CAS No.:51803-78-2
- Oxoepistephamiersine
Catalog No.:BCN5645
CAS No.:51804-68-3
- Dihydrooxoepistephamiersine
Catalog No.:BCN5646
CAS No.:51804-69-4
- Raltegravir (MK-0518)
Catalog No.:BCC2137
CAS No.:518048-05-0
- KX1-004
Catalog No.:BCC5440
CAS No.:518058-84-9
- 2',4'-Dihydroxychalcone
Catalog No.:BCN5647
CAS No.:1776-30-3
- UMI-77
Catalog No.:BCC5567
CAS No.:518303-20-3
- Angiotensin 1/2 + A (2 - 8)
Catalog No.:BCC1037
CAS No.:51833-76-2
- Angiotensin (1-7)
Catalog No.:BCC1029
CAS No.:51833-78-4
- 3-MATIDA
Catalog No.:BCC7281
CAS No.:518357-51-2
Synthesis of (aminoalkyl)cycleanine analogues: cytotoxicity, cellular uptake, and apoptosis induction in ovarian cancer cells.[Pubmed:29588214]
Bioorg Med Chem Lett. 2018 May 15;28(9):1652-1656.
Our previous studies demonstrated that Cycleanine, a macrocyclic bisbenzylisoquinoline (BBIQ) alkaloid, showed potent anti-ovarian cancer activity via apoptosis induction. Here, we synthesized two novel (aminoalkyl)Cycleanine analogues (2 and 3) through a simple and efficient two-step reaction starting from Cycleanine isolated from Triclisia subcordata Oliv. These analogues showed greater potency than the unmodified Cycleanine in three human ovarian cancer cell lines. Both 2 and 3 induced apoptosis in ovarian cancer cells by activations of caspases 3/7, cleavage of PARP, increase in subG1 cell cycle phase and in the percentage of apoptotic cells. Further confocal fluorescence microscopy analysis confirmed the cellular uptake of alkaloids in ovarian cancer cells by using the unique (alkynyl)Cycleanine (3) via click chemistry reaction. Our results suggest that Cycleanine could be a hit compound for the future development in attacking ovarian cancer.
Anti-malarial Activity of Isoquinoline Alkaloids from the Stem Bark of Actinodaphne macrophylla.[Pubmed:26594753]
Nat Prod Commun. 2015 Sep;10(9):1541-2.
Seven isoquinoline alkaloids isolated from the bark of Actinodaphne macrophylla in this study demonstrated in vitro antiplasmodial activities against Plasmodium falciparum 3D7 with IC50 values of 0.08 microM, 0.05 microM, 1.18 microM, 3.11 microM, 0.65 microM, 0.26 microM, and 1.38 microM for Cycleanine, 10-demethylxylopinine, reticuline, laurotetanine, bicuculine, alpha-hydrastine and anolobine, respectively, which are comparable with the reference standard, chloroquine. 10-Demethylxylopinine was found to be the most active of these compounds.
Bioactive isoquinoline alkaloids from Cissampelos pareira.[Pubmed:29126362]
Nat Prod Res. 2017 Nov 10:1-6.
The phytochemical and biological investigation of Cissampelos pareira leads to the isolation of one new isoquinoline alkaloid (7) along with six known isoquinoline alkaloids, namely, magnoflorine (1), magnocurarine (2), cissamine (3), curine (4), hayatinine (5) and Cycleanine (6). Magnoflorine (1) and magnocurarine (2) were isolated for the first time from C. pareira. A new, rapid, simple and sensitive UPLC method was developed for simultaneous quantification of five pure compounds (1-5). Seasonal variation study revealed higher content of these compounds during the rainy season. The chloroform (CPCF) and n-butanol (CPBF) fractions showed cytotoxic efficacy against KB cells. Among pure compounds, hayatinine (5) was found to be most active against KB and A549, while, Cycleanine (6) against KB cells.
Interaction of cepharanthine with immobilized heat shock protein 90alpha (Hsp90alpha) and screening of Hsp90alpha inhibitors.[Pubmed:23219559]
Anal Biochem. 2013 Mar 1;434(1):202-6.
Heat shock protein 90alpha (Hsp90alpha) immobilized on aminopropyl silica gels was prepared via the N- or C-terminal, which was termed Hsp90alpha-NT or Hsp90alpha-CT, respectively. Binding interactions of biscoclaurine alkaloids (cepharanthine (CEP), berbamine (BBM), isotetrandrine (ITD), and Cycleanine (CCN)) with Hsp90alpha were examined using the Hsp90alpha-NT or -CT columns by frontal and zonal chromatography studies. The dissociation constants of CEP, BBM, ITD, and CCN to Hsp90alpha-NT were estimated to be 5.3, 18.6, 46.3, and 159 muM, respectively, by frontal chromatography techniques. Similar results were obtained with the Hsp90alpha-CT column. These data suggest that these biscoclaurine alkaloids interact with the middle domain of Hsp90alpha. This was confirmed by demonstrating that CEP competed with endothelial nitric oxide synthase at the middle domain of Hsp90alpha, where it was shown to have a dissociation constant of 15 nM. Furthermore, the Hsp90alpha-NT column was applied for preliminary screening of natural Hsp90alpha inhibitors by zonal chromatography studies.
Antileishmanial, antitrypanosomal, and cytotoxic screening of ethnopharmacologically selected Peruvian plants.[Pubmed:21922239]
Parasitol Res. 2012 Apr;110(4):1381-92.
Extracts (34) from eight plant species of the Peruvian Amazonia currently used in traditional Peruvian medicine, mostly as antileishmanial remedies and also as painkiller, antiseptic, antipyretic, anti-inflamatory, antiflu, astringent, diuretic, antipoison, anticancerous, antiparasitic, insecticidal, or healing agents, have been tested for their antileishmanial, antitrypanosomal, and cytotoxic activity. Plant species were selected based on interviews conducted with residents of rural areas. The different plant parts were dried, powdered, and extracted by maceration with different solvents (hexane, chloroform, and 70% ethanol-water). These extracts were tested on promastigote forms of Leishmania infantum strain PB75, epimastigote forms of Trypanosoma cruzi strain Y, and the mammalian CHO cell line. Parasite viability and nonspecific cytotoxicity were analyzed by a modified MTT colorimetric assay method. The isolation and identification of pure compounds from selected extracts were performed by column chromatography, gas chromatography mass spectrometry (GC-MS; mixtures), spectroscopic techniques [MS, infrared (IR), ultraviolet (UV)], and mono and two-dimensional (1)H and (13)C nuclear magnetic resonance (NMR; COSY, HSQC, NOESY) experiments. Chondodendron tomentosum bark and Cedrela odorata were the most active extracts against Leishmania, while C. odorata and Aristoloquia pilosa were the most active against Trypanosoma, followed by Tabebuia serratifolia, Tradescantia zebrina, and Zamia ulei. Six compounds and two mixtures were isolated from Z. ulei [cycasin (1)], T. serratifolia {mixtures 1-2, and naphthoquinones 2-acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione (2) and 2-(1-hydroxyethyl)-4H,9H-naphtho[2,3-b]furan-4,9-dione (3)}, and C. tomentosum [chondrocurine (4); (S,S')-12-O-methyl(+)-curine (5); and Cycleanine (6)]. Four compounds and the two mixtures exhibited significant activity.
Cytotoxicity Effects and Apoptosis Induction by Bisbenzylisoquinoline Alkaloids from Triclisia subcordata.[Pubmed:27270992]
Phytother Res. 2016 Sep;30(9):1533-9.
Triclisia subcordata Oliv (Menispermeaceae) is a medicinal plant traditionally used for the treatment of various diseases in West Africa. The ethanol extract of T. subcordata and its fractions were screened for in vitro anti-ovarian cancer activities using the Sulforhodamine B assay. The crude alkaloids showed the strongest activity in cell growth assays on Ovcar-8 and A2780 cell lines (IC50 < 2.4 microg/mL). A bisbenzylisoquinoline alkaloid-Cycleanine was isolated using HPLC and identified by mass spectrometry and nuclear magnetic resonance analyses. The IC50 values of Cycleanine and tetrandrine (an alkaloid previously reported from this plant) ranged from 7 to 14 muM on Ovcar-8, A2780, Ovcar-4, and Igrov-1 ovarian cancer cell lines. The IC50 of Cycleanine on human normal ovarian surface epithelial cells was 35 +/- 1 muM, hinting at modest selectivity toward cancer cells. Both Cycleanine and tetrandrine caused apoptosis as shown by activation of caspases 3/7 and cleavage of poly(ADP-ribose) polymerase to form poly(ADP-ribose) polymerase-1 by using western blot analysis. Flow cytometry analyses showed that the percentages of apoptotic cells and cells in subG1 phase increased after exposure of Cycleanine and tetrandrine to Ovcar-8 cells for 48 h compared with control. Cycleanine, like its isomer tetrandrine isolated from T. subcordata, could be a potential new anti-ovarian cancer agent acting through the apoptosis pathway. Copyright (c) 2016 John Wiley & Sons, Ltd.


