CorymbosinCAS# 18103-41-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18103-41-8 | SDF | Download SDF |
| PubChem ID | 10970376 | Appearance | Powder |
| Formula | C19H18O7 | M.Wt | 358.34 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
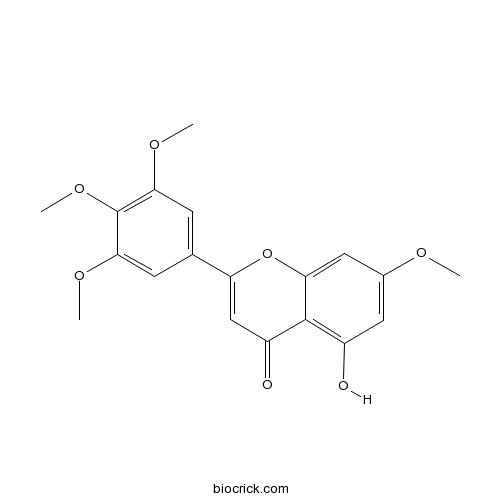
| Chemical Name | 5-hydroxy-7-methoxy-2-(3,4,5-trimethoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC(=C2C(=C1)OC(=CC2=O)C3=CC(=C(C(=C3)OC)OC)OC)O | ||
| Standard InChIKey | FLCVGMVLNHYJAW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18O7/c1-22-11-7-12(20)18-13(21)9-14(26-15(18)8-11)10-5-16(23-2)19(25-4)17(6-10)24-3/h5-9,20H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Corymbosin shows significant antiviral activity. |
| Targets | Antifection |

Corymbosin Dilution Calculator

Corymbosin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7906 mL | 13.9532 mL | 27.9065 mL | 55.8129 mL | 69.7661 mL |
| 5 mM | 0.5581 mL | 2.7906 mL | 5.5813 mL | 11.1626 mL | 13.9532 mL |
| 10 mM | 0.2791 mL | 1.3953 mL | 2.7906 mL | 5.5813 mL | 6.9766 mL |
| 50 mM | 0.0558 mL | 0.2791 mL | 0.5581 mL | 1.1163 mL | 1.3953 mL |
| 100 mM | 0.0279 mL | 0.1395 mL | 0.2791 mL | 0.5581 mL | 0.6977 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Curcumaromin C
Catalog No.:BCN7418
CAS No.:1810034-40-2
- Curcumaromin B
Catalog No.:BCN7419
CAS No.:1810034-39-9
- Curcumaromin A
Catalog No.:BCN7417
CAS No.:1810034-38-8
- 3-O-Caffeoylshikimic acid
Catalog No.:BCN7930
CAS No.:180981-12-8
- FTI 277 HCl
Catalog No.:BCC6395
CAS No.:180977-34-8
- Neuchromenin
Catalog No.:BCN7449
CAS No.:180964-26-5
- Spiramilactone B
Catalog No.:BCN1141
CAS No.:180961-65-3
- Jaceosidin
Catalog No.:BCN2529
CAS No.:18085-97-7
- 4-O-Caffeoylshikimic acid
Catalog No.:BCN7931
CAS No.:180842-65-3
- CHMFL-ABL-053
Catalog No.:BCC3988
CAS No.:1808287-83-3
- LG 100754
Catalog No.:BCC7786
CAS No.:180713-37-5
- Polypodine B
Catalog No.:BCN8117
CAS No.:18069-14-2
- 5,7-Dihydroxy-3',4',5'-trimethoxyflavone
Catalog No.:BCN6807
CAS No.:18103-42-9
- Proflavine Hemisulfate
Catalog No.:BCC4707
CAS No.:1811-28-5
- 5-Hydroxy-4-methoxycanthin-6-one
Catalog No.:BCN1142
CAS No.:18110-86-6
- 4,5-Dimethoxycanthin-6-one
Catalog No.:BCN1143
CAS No.:18110-87-7
- Co 102862
Catalog No.:BCC7439
CAS No.:181144-66-1
- Almotriptan Malate
Catalog No.:BCC5045
CAS No.:181183-52-8
- DEL-22379
Catalog No.:BCC6521
CAS No.:181223-80-3
- Ginsenoside F4
Catalog No.:BCN2881
CAS No.:181225-33-2
- Taxuspine W
Catalog No.:BCN6937
CAS No.:181309-92-2
- Colutehydroquinone
Catalog No.:BCN8239
CAS No.:181311-16-0
- PD 160170
Catalog No.:BCC7284
CAS No.:181468-88-2
- Isonemerosin
Catalog No.:BCN6550
CAS No.:181524-79-8
How to deal with nomenclatoral ambiguities of trivial names for natural products?--a clarifying case study exemplified for "corymbosin".[Pubmed:24660463]
Nat Prod Commun. 2014 Jan;9(1):57-60.
Many names of plant secondary compounds that have been isolated and identified in the course of phytochemical investigations are based either on the vernacular or Latin names of the source plants, are constructed according to rules of chemical nomenclature, or consist of in-between forms. Trivial names, based on the specific epithets of biological organisms, occasionally create confusion because such epithets are used in numerous combinations and, therefore, could potentially be used when naming chemical entities from radically different sources. Such an example of ambiguous naming is represented with the case of Corymbosin, a name that was assigned to two chemically distinct compounds that were isolated and reported simultaneously in 1967 from two different spermatophyte taxa: a terpene glucoside from Turbina corymbosa and a flavone from Webera corymbosa. The flavone is more widespread and has been reported so far from 15 taxa, whereas the glucoside has thus far only been isolated from the original source species. Furthermore, glycosides named Corymbosins K1-K4 were isolated in 2006 from Knoxia corymbosa. This article emphasizes the need to adhere to strict principles when naming secondary constituents and suggests that a practice should be applied that is similar to the application of the priority rules used in botanical nomenclature for homonyms. The use of the trivial name, Corymbosin, should be applied only to the more widespread tricetin-7,3',4',5'-tetramethyl ether by rules of conservation.
Chromone glycosides from Knoxia corymbosa.[Pubmed:17135054]
J Asian Nat Prod Res. 2006 Oct-Nov;8(7):663-70.
Four new chromone glycosides, Corymbosins K1-K4 (3-6), together with two known compounds, noreugenin (1) and undulatoside A (2), were isolated from the whole plant of Knoxiacorymbosa (Rubiaceae). The structures of the new compounds were established through extensive NMR or X-ray spectroscopic analysis as 7-O-beta-D-allopyranosyl-5-hydroxy-2-methylchromone (Corymbosin K1, 3), 7-O-beta-D-6-acetylglucopyranosyl-5-hydroxy-2-methylchromone (Corymbosin K2, 4), 7-O-[6-O-(4-O-trans-caffeoyl-beta-D-allopyranosyl)]-beta-D-glucopyranosyl-5-hydro xy-2-methylchromone (Corymbosin K3, 5) and 7-O-[6-O-(4-O-trans-feruloyl-beta-D-allopyranosyl)]-beta-D-glucopyranosyl-5-hydro xy-2- methylchromone (Corymbosin K4, 6). Compounds 2-5 were subjected to test their immunomodulatory activity invitro.


