Cerebroside BCAS# 88642-46-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 88642-46-0 | SDF | Download SDF |
| PubChem ID | 11498616 | Appearance | Powder |
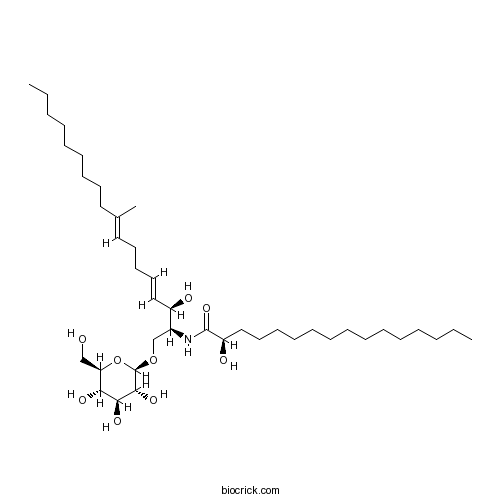
| Formula | C41H77NO9 | M.Wt | 728.1 |
| Type of Compound | Cerebrosides | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R)-2-hydroxy-N-[(2S,3R,4E,8E)-3-hydroxy-9-methyl-1-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoctadeca-4,8-dien-2-yl]hexadecanamide | ||
| SMILES | CCCCCCCCCCCCCCC(C(=O)NC(COC1C(C(C(C(O1)CO)O)O)O)C(C=CCCC=C(C)CCCCCCCCC)O)O | ||
| Standard InChIKey | YBSQGNFRWZKFMJ-FRJHFHMPSA-N | ||
| Standard InChI | InChI=1S/C41H77NO9/c1-4-6-8-10-12-13-14-15-16-18-20-24-29-35(45)40(49)42-33(31-50-41-39(48)38(47)37(46)36(30-43)51-41)34(44)28-25-21-23-27-32(3)26-22-19-17-11-9-7-5-2/h25,27-28,33-39,41,43-48H,4-24,26,29-31H2,1-3H3,(H,42,49)/b28-25+,32-27+/t33-,34+,35+,36+,37+,38-,39+,41+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1.Cerebroside B1b has antiulcerogenic activity. |
| Targets | Antifection |

Cerebroside B Dilution Calculator

Cerebroside B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3734 mL | 6.8672 mL | 13.7344 mL | 27.4688 mL | 34.3359 mL |
| 5 mM | 0.2747 mL | 1.3734 mL | 2.7469 mL | 5.4938 mL | 6.8672 mL |
| 10 mM | 0.1373 mL | 0.6867 mL | 1.3734 mL | 2.7469 mL | 3.4336 mL |
| 50 mM | 0.0275 mL | 0.1373 mL | 0.2747 mL | 0.5494 mL | 0.6867 mL |
| 100 mM | 0.0137 mL | 0.0687 mL | 0.1373 mL | 0.2747 mL | 0.3434 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chrysin 7-O-beta-gentiobioside
Catalog No.:BCN2943
CAS No.:88640-89-5
- LY2119620
Catalog No.:BCC5564
CAS No.:886047-22-9
- Isomexoticin
Catalog No.:BCN4434
CAS No.:88585-86-8
- Fmoc-ε-Acp-OH
Catalog No.:BCC3206
CAS No.:88574-06-5
- c-FMS inhibitor
Catalog No.:BCC1472
CAS No.:885704-21-2
- 5'-O-Acetyljuglanin
Catalog No.:BCN6846
CAS No.:885697-82-5
- CCT128930
Catalog No.:BCC3904
CAS No.:885499-61-6
- Minumicrolin
Catalog No.:BCN4433
CAS No.:88546-96-7
- HJC 0350
Catalog No.:BCC6302
CAS No.:885434-70-8
- MK-8745
Catalog No.:BCC3994
CAS No.:885325-71-3
- Kongensin A
Catalog No.:BCN4432
CAS No.:885315-96-8
- W-13 hydrochloride
Catalog No.:BCC6620
CAS No.:88519-57-7
- Apogossypolone (ApoG2)
Catalog No.:BCC2237
CAS No.:886578-07-0
- 11-Hydroxy-sugiol
Catalog No.:BCN2510
CAS No.:88664-08-8
- 14-Deoxycoleon U
Catalog No.:BCN4796
CAS No.:88664-09-9
- 8-Epideoxyloganic acid
Catalog No.:BCN6576
CAS No.:88668-99-9
- Liranaftate
Catalog No.:BCC4672
CAS No.:88678-31-3
- Piperlotine C
Catalog No.:BCN6485
CAS No.:886989-88-4
- Neobritannilactone B
Catalog No.:BCN3510
CAS No.:886990-00-7
- Borneol 7-O-[beta-D-apiofuranosyl-(1->6)]-beta-D-glucopyranoside
Catalog No.:BCN7814
CAS No.:88700-35-0
- 1alpha-Hydroxytorilin
Catalog No.:BCN6648
CAS No.:887147-75-3
- Adynerigenin beta-neritrioside
Catalog No.:BCN4556
CAS No.:88721-09-9
- Epiglobulol
Catalog No.:BCN7121
CAS No.:88728-58-9
- Hebeirubescensin H
Catalog No.:BCN7155
CAS No.:887333-30-4
Delta10(E)-Sphingolipid Desaturase Involved in Fusaruside Mycosynthesis and Stress Adaptation in Fusarium graminearum.[Pubmed:25994332]
Sci Rep. 2015 May 21;5:10486.
Sphingolipids are biologically important and structurally distinct cell membrane components. Fusaruside (1) is a 10,11-unsaturated immunosuppressive fungal sphingolipid with medical potentials for treating liver injury and colitis, but its poor natural abundance bottlenecks its druggability. Here, fusaruside is clarified biosynthetically, and its efficacy-related 10,11-double bond can be generated under the regioselective catalysis of an unprecedented Delta10(E)-sphingolipid desaturase (Delta10(E)-SD). Delta10(E)-SD shares 17.7% amino acid sequence similarity with a C9-unmethylated Delta10-sphingolipid desaturase derived from a marine diatom, and 55.7% with Delta8(E)-SD from Fusarium graminearum. Heterologous expression of Delta10(E)-SD in Pichia pastoris has been established to facilitate a reliable generation of 1 through the Delta10(E)-SD catalyzed desaturation of Cerebroside B (2), an abundant fungal sphingolipid. Site directed mutageneses show that the conserved histidines of Delta10(E)-SD are essential for the 10,11-desaturation catalysis, which is also preconditioned by the C9-methylation of the substrate. Moreover, Delta10(E)-SD confers improved survival and faster growth to fungal strains at low temperature and high salinity, in parallel with to higher contents of 1 in the mycelia. Collectively, the investigation describes a new Delta10(E)-sphingolipid desaturase with its heterologous expression fundamentalizing a biotechnological supply of 1, and eases the follow-up clarification of the immunosuppression and stress-tolerance mechanism.
Steroids and phenolic constituents from the fruiting bodies of the basidiomycete Sarcodon joedes.[Pubmed:22320163]
Nat Prod Res. 2013;27(1):80-4.
Nine secondary metabolites, including four steroids, four phenolics and one cerebroside, were isolated from the methanol extract of the fruiting bodies of the basidiomycete Sarcodon joedes. The isolated compounds were identified by spectroscopic analyses as (22E,24R)-6beta-methoxyergosta-7,22-diene-3beta,5alpha-diol (1), 2',3'-diacetoxy-3,4,5',6',4''-pentahydroxy-p-terphenyl (2), Cerebroside B (3), ergosta-7,22-dien-3beta-ol (4), ergosterol peroxide (5), (22E,24R)-3beta-hydroxy-ergosta-5,22-dien-7-one (6), benzoic acid (7), methyl p-hydroxybenzoate (8) and 3,4-dihydroxybenzoic acid (9). The cytotoxic activities of these compounds were evaluated. All these compounds were isolated from this fungus for the first time.


