8-Epideoxyloganic acidCAS# 88668-99-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 88668-99-9 | SDF | Download SDF |
| PubChem ID | 443332 | Appearance | Powder |
| Formula | C16H24O9 | M.Wt | 360.4 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
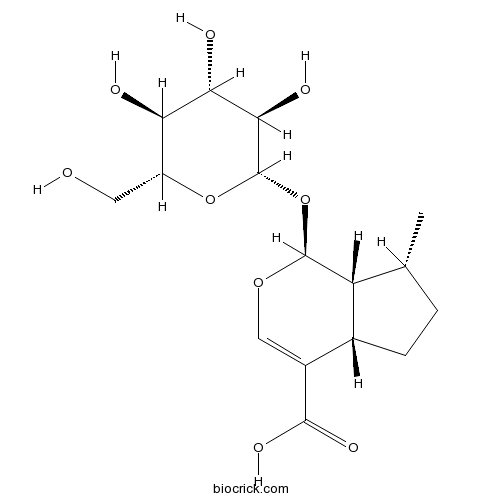
| Chemical Name | (1S,4aS,7R,7aR)-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-4-carboxylic acid | ||
| SMILES | CC1CCC2C1C(OC=C2C(=O)O)OC3C(C(C(C(O3)CO)O)O)O | ||
| Standard InChIKey | DSXFHNSGLYXPNG-PKUPRILXSA-N | ||
| Standard InChI | InChI=1S/C16H24O9/c1-6-2-3-7-8(14(21)22)5-23-15(10(6)7)25-16-13(20)12(19)11(18)9(4-17)24-16/h5-7,9-13,15-20H,2-4H2,1H3,(H,21,22)/t6-,7-,9-,10-,11-,12+,13-,15+,16+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 8-Epideoxyloganic acid (oral) shows weak antinociceptive activity. 2. 8-Epideoxyloganic acid has the potential to serve as anti-inflammatory agents during oxidative stress, the inhibition of ROS production, possibly through modulation of NOX activity and/or the radical scavenging effect, and beta2 integrin expression in leucocytes. 3. 8-Epideoxyloganic acid possesses bioactivities of analgesia, homeostasis and anti-inflammatory. |
| Targets | Immunology & Inflammation related | ROS |

8-Epideoxyloganic acid Dilution Calculator

8-Epideoxyloganic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7747 mL | 13.8735 mL | 27.7469 mL | 55.4939 mL | 69.3674 mL |
| 5 mM | 0.5549 mL | 2.7747 mL | 5.5494 mL | 11.0988 mL | 13.8735 mL |
| 10 mM | 0.2775 mL | 1.3873 mL | 2.7747 mL | 5.5494 mL | 6.9367 mL |
| 50 mM | 0.0555 mL | 0.2775 mL | 0.5549 mL | 1.1099 mL | 1.3873 mL |
| 100 mM | 0.0277 mL | 0.1387 mL | 0.2775 mL | 0.5549 mL | 0.6937 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 14-Deoxycoleon U
Catalog No.:BCN4796
CAS No.:88664-09-9
- 11-Hydroxy-sugiol
Catalog No.:BCN2510
CAS No.:88664-08-8
- Apogossypolone (ApoG2)
Catalog No.:BCC2237
CAS No.:886578-07-0
- Cerebroside B
Catalog No.:BCN4435
CAS No.:88642-46-0
- Chrysin 7-O-beta-gentiobioside
Catalog No.:BCN2943
CAS No.:88640-89-5
- LY2119620
Catalog No.:BCC5564
CAS No.:886047-22-9
- Isomexoticin
Catalog No.:BCN4434
CAS No.:88585-86-8
- Fmoc-ε-Acp-OH
Catalog No.:BCC3206
CAS No.:88574-06-5
- c-FMS inhibitor
Catalog No.:BCC1472
CAS No.:885704-21-2
- 5'-O-Acetyljuglanin
Catalog No.:BCN6846
CAS No.:885697-82-5
- CCT128930
Catalog No.:BCC3904
CAS No.:885499-61-6
- Minumicrolin
Catalog No.:BCN4433
CAS No.:88546-96-7
- Liranaftate
Catalog No.:BCC4672
CAS No.:88678-31-3
- Piperlotine C
Catalog No.:BCN6485
CAS No.:886989-88-4
- Neobritannilactone B
Catalog No.:BCN3510
CAS No.:886990-00-7
- Borneol 7-O-[beta-D-apiofuranosyl-(1->6)]-beta-D-glucopyranoside
Catalog No.:BCN7814
CAS No.:88700-35-0
- 1alpha-Hydroxytorilin
Catalog No.:BCN6648
CAS No.:887147-75-3
- Adynerigenin beta-neritrioside
Catalog No.:BCN4556
CAS No.:88721-09-9
- Epiglobulol
Catalog No.:BCN7121
CAS No.:88728-58-9
- Hebeirubescensin H
Catalog No.:BCN7155
CAS No.:887333-30-4
- K-115
Catalog No.:BCC5500
CAS No.:887375-67-9
- 3,9-Dihydroeucomin
Catalog No.:BCN6832
CAS No.:887375-68-0
- INCB 3284 dimesylate
Catalog No.:BCC1646
CAS No.:887401-93-6
- Fmoc-Arg(Mts)-OH
Catalog No.:BCC3075
CAS No.:88743-97-9
The inhibitory effect of phenylpropanoid glycosides and iridoid glucosides on free radical production and beta2 integrin expression in human leucocytes.[Pubmed:16393473]
J Pharm Pharmacol. 2006 Jan;58(1):129-35.
Rapid production of reactive oxygen species (ROS) and upregulation of beta2 integrin by leucocytes are two important inflammatory responses in human leucocytes. To evaluate whether three phenylpropanoid glycosides (acteoside, crenatoside, and rossicaside B) and two iridoid glucosides (boschnaloside and 8-Epideoxyloganic acid) identified from two medicinal plants with similar indications (Orobanche caerulescens and Boschniakia rossica) exhibited anti-inflammatory activity, their effects on N-formyl-methionyl-leucyl-phenylalanine (fMLP) and phorbol-12-myristate-13-acetate (PMA)-activated peripheral human neutrophils (PMNs) and mononuclear cells were examined. Pretreatment with 1-50 microM phenylpropanoid glycoside concentration-dependently diminished PMA- and fMLP-induced ROS production with IC50 values of approximately 6.8-23.9 and 3.0-8.8 muM, respectively. Iridoid glucoside was less effective than phenylpropanoid glycoside with an IC50 value of approximately 8.9-28.4 microM in PMA-activated PMNs and 19.1-21.1 microM in fMLP-activated mononuclear cells. Phenylpropanoid glycosides also effectively inhibited NADPH oxidase (NOX) and displayed potent free radical-scavenging activity, but did not interfere with pan-protein kinase C (PKC) activity. Furthermore, all compounds, except rossicaside B, significantly inhibited PMA- and fMLP-induced Mac-1 (a beta2 integrin) upregulation at 50 microM but not that of fMLP-induced intracellular calcium mobilization. These drugs had no significant cytotoxicity as compared with the vehicle control. Our data suggested that inhibition of ROS production, possibly through modulation of NOX activity and/or the radical scavenging effect, and beta2 integrin expression in leucocytes indicated that these compounds had the potential to serve as anti-inflammatory agents during oxidative stress.
[Constituents and bioactivities of Lamiophlomis rotata].[Pubmed:21598543]
Zhongguo Zhong Yao Za Zhi. 2011 Feb;36(4):465-7.
OBJECTIVE: To investigate the chemical constituents from Lamiophlomis rotata and the bioactivities of 8-Epideoxyloganic acid. METHOD: The constituents were isolated by using a combination of various chromatographic techniques including column chromatography over ployamide, silica gel and Sephadex LH-20. Structures of the isolates were identified by spectroscopic data analysis. Bioactivities were screened by using models in vivo. RESULT: Five constituents were isolated. 8-Epideoxyloganic acid was isolated for the first time in L. rotata and also in lamioplomis genus. 8-Epideoxyloganic acid could significantly inhibit aectic acid-induced twisting times and slower the time of homeostatsis, also inhibit xylene-induced ear edema in mice. CONCLUSION: 8-Epideoxyloganic acid possesses bioactivities of analgesia, homeostasis and anti-inflammatory.
Antinociceptive substances from Incarvillea delavayi.[Pubmed:10680179]
Phytochemistry. 2000 Jan;53(2):253-6.
Antinociceptive activities of an Incarvillea delavayi extract, as well as its constituents, 8-Epideoxyloganic acid and delavayine A, were evaluated in the acetic acid induced writhing test in mice. An oral administration of the delavayi extract weakly decreased the number of writhings and stretchings in this test, in a dose-dependent manner. Furthermore, orally administered 8-Epideoxyloganic acid showed weak antinociceptive activity, whereas administration by subcutaneous injection did not. However, subcutaneous injection of delavayine A, a novel monoterpene alkaloid, showed a more significant level of antinociceptive activity.


