CedrolCAS# 77-53-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 77-53-2 | SDF | Download SDF |
| PubChem ID | 65575 | Appearance | Colorless crystal |
| Formula | C15H26O | M.Wt | 222.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
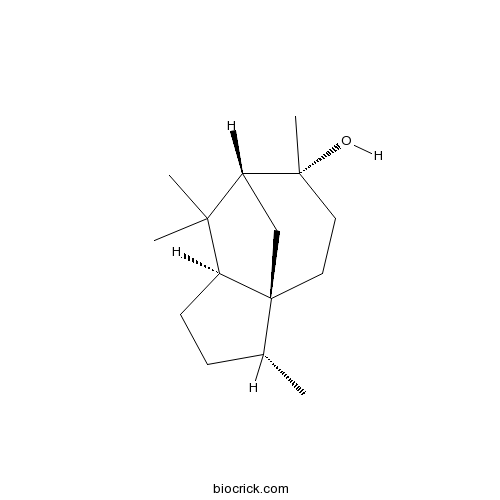
| Chemical Name | (1S,2R,5S,7R,8R)-2,6,6,8-tetramethyltricyclo[5.3.1.01,5]undecan-8-ol | ||
| SMILES | CC1CCC2C13CCC(C(C3)C2(C)C)(C)O | ||
| Standard InChIKey | SVURIXNDRWRAFU-OGMFBOKVSA-N | ||
| Standard InChI | InChI=1S/C15H26O/c1-10-5-6-11-13(2,3)12-9-15(10,11)8-7-14(12,4)16/h10-12,16H,5-9H2,1-4H3/t10-,11+,12-,14-,15+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cedrol Dilution Calculator

Cedrol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.497 mL | 22.485 mL | 44.9701 mL | 89.9402 mL | 112.4252 mL |
| 5 mM | 0.8994 mL | 4.497 mL | 8.994 mL | 17.988 mL | 22.485 mL |
| 10 mM | 0.4497 mL | 2.2485 mL | 4.497 mL | 8.994 mL | 11.2425 mL |
| 50 mM | 0.0899 mL | 0.4497 mL | 0.8994 mL | 1.7988 mL | 2.2485 mL |
| 100 mM | 0.045 mL | 0.2249 mL | 0.4497 mL | 0.8994 mL | 1.1243 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ursolic acid
Catalog No.:BCN4327
CAS No.:77-52-1
- Chlorthalidone
Catalog No.:BCC4649
CAS No.:77-36-1
- Gibberellins
Catalog No.:BCN2189
CAS No.:77-06-5
- Garcinone C
Catalog No.:BCN4322
CAS No.:76996-27-5
- Euphroside
Catalog No.:BCN6633
CAS No.:76994-07-5
- Notoginsenoside T5
Catalog No.:BCN3727
CAS No.:769932-34-5
- 15-Methoxypinusolidic acid
Catalog No.:BCN4321
CAS No.:769928-72-5
- TPT-260
Catalog No.:BCC5171
CAS No.:769856-81-7
- Cleomiscosin B
Catalog No.:BCN3898
CAS No.:76985-93-8
- Boc-D-2-Nal-OH
Catalog No.:BCC3288
CAS No.:76985-10-9
- H-D-2-Nal-OH.HCl
Catalog No.:BCC3286
CAS No.:76985-09-6
- Nizatidine
Catalog No.:BCC4522
CAS No.:76963-41-2
- Tomatidine
Catalog No.:BCN2773
CAS No.:77-59-8
- Tigogenin
Catalog No.:BCN5327
CAS No.:77-60-1
- Trometamol
Catalog No.:BCC4743
CAS No.:77-86-1
- Citric acid
Catalog No.:BCN5374
CAS No.:77-92-9
- Triethyl citrate
Catalog No.:BCC9186
CAS No.:77-93-0
- D-(-)-Quinic acid
Catalog No.:BCN1029
CAS No.:77-95-2
- (+,-)-Octopamine HCl
Catalog No.:BCC4814
CAS No.:770-05-8
- Dehydropachymic acid
Catalog No.:BCN3648
CAS No.:77012-31-8
- Hypocrellin A
Catalog No.:BCN3396
CAS No.:77029-83-5
- Knightinol
Catalog No.:BCN1913
CAS No.:77053-06-6
- Acetylknightinol
Catalog No.:BCN1914
CAS No.:77053-07-7
- Gadobutrol
Catalog No.:BCC4164
CAS No.:770691-21-9
Silicon Supplementation Alters the Composition of Herbivore Induced Plant Volatiles and Enhances Attraction of Parasitoids to Infested Rice Plants.[Pubmed:28769965]
Front Plant Sci. 2017 Jul 19;8:1265.
Silicon (Si) is important in plant defenses that operate in a direct manner against herbivores, and work in rice (Oryza sativa) has established that this is mediated by the jasmonate signaling pathway. Plant defenses also operate indirectly, by the production of herbivore induced plant volatiles (HIPVs) that attract predators and parasitoids of herbivores. These indirect defenses too are mediated by the jasmonate pathway but no earlier work has demonstrated an effect of Si on HIPVs. In this study, we tested the effect of Si supplementation versus Si deprivation to rice plants on subsequent HIPV production following feeding by the important pest, rice leaffolder (Cnaphalocrocis medinalis). Gas chromatography-mass spectrometry analyses showed lower production of alpha-bergamotene, beta-sesquiohellandrene, hexanal 2-ethyl, and Cedrol from +Si herbivore-infested plants compared with -Si infested plants. These changes in plant chemistry were ecologically significant in altering the extent to which parasitoids were attracted to infested plants. Adult females of Trathala flavo-orbitalis and Microplitis mediator both exhibited greater attraction to the HIPV blend of +Si plants infested with their respective insect hosts compared to -Si infested plants. In equivalent studies using RNAi rice plants in which jasmonate perception was silenced there was no equivalent change to the HIPV blend associated with Si treatment; indicating that the effects of Si on HIPVs are modulated by the jasmonate pathway. Further, this work demonstrates that silicon alters the HIPV blend of herbivore-infested rice plants. The significance of this finding is that there are no earlier-published studies of this phenomenon in rice or any other plant species. Si treatment to crops offers scope for enhancing induced, indirect defenses and associated biological control of pests because parasitoids are more strongly attracted by the HIPVs produced by +Si plants.
Total Syntheses of (-)-Majucin and (-)-Jiadifenoxolane A, Complex Majucin-Type Illicium Sesquiterpenes.[Pubmed:29148748]
J Am Chem Soc. 2017 Dec 13;139(49):17783-17786.
We report the first chemical syntheses of both (-)-majucin and (-)-jiadifenoxolane A via 10 net oxidations from the ubiquitous terpene (+)-Cedrol. Additionally, this approach allows for access to other majucin-type sesquiterpenes, like (-)-jiadifenolide, (-)-jiadifenin, and (-)-(1R,10S)-2-oxo-3,4-dehydroxyneomajucin (ODNM) along the synthetic pathway. Site-selective aliphatic C(sp(3))-H bond oxidation reactions serve as the cornerstone of this work which offers access to highly oxidized natural products from an abundant and renewable terpene feedstock.
Inhibitory effect of a new orally active cedrol-loaded nanostructured lipid carrier on compound 48/80-induced mast cell degranulation and anaphylactic shock in mice.[Pubmed:28744120]
Int J Nanomedicine. 2017 Jul 7;12:4849-4868.
BACKGROUND: Type I hypersensitivity is an allergic reaction characterized by the overactivity of the immune system provoked by normally harmless substances. Glucocorticoids, anti-histamines, or mast cell stabilizers are the choices of treatment for type I hypersensitivity. Even though these drugs have the anti-allergic effect, they can have several side effects in prolong use. Cedrol is the main bioactive compound of Cedrus atlantica with anti-tumor, anti-oxidative, and platelet-activating factor inhibiting properties. METHODS: In this study, the preparation and anti-anaphylactic effect of Cedrol-loaded nanostructured lipid carriers (NLCs) were evaluated. NLCs were prepared using Compritol((R)) 888 ATO and triolein as lipid phase and vitamin E d-alpha-tocopherylpolyethyleneglycol 1000 succinate, soya lecithin, and sodium deoxycholate as nanoparticle stabilizers. RESULTS: The average diameter of Cedrol-NLCs (CR-NLCs) was 71.2 nm (NLC-C1) and 91.93 nm (NLC-C2). The particle had negative zeta potential values of -31.9 mV (NLC-C1) and -44.5 mV (NLC-C2). Type I anaphylactoid reaction in the animal model is significantly reduced by Cedrol and Cedrol-NLC. This in vivo activity of Cedrol resulted that Cedrol suppressed compound 48/80-induced peritoneal mast cell degranulation and histamine release from mast cells. Furthermore, compound 48/80-evoked Ca(2+) uptake into mast cells was reduced in a dose-dependent manner by Cedrol and Cedrol-NLC. Studies confirmed that the inhibition of type I anaphylactoid response in vivo in mice and compound 48/80-induced mast cell activation in vitro are greatly enhanced by the loading of Cedrol into the NLCs. The safety of Cedrol and CR-NLC was evaluated as selectivity index (SI) with prednisolone and cromolyn sodium as positive control. SI of CR-NLC-C2 was found to be 11.5-fold greater than both prednisolone and cromolyn sodium. CONCLUSION: Administration of CR-NLC 24 hours before the onset of anaphylaxis can prevent an anaphylactoid reaction. NLCs could be a promising vehicle for the oral delivery of Cedrol to protect anaphylactic reactions.
Variation of volatile terpenes in the edible fungi mycelia Flammulina velutipes and communications in fungus-mite interactions.[Pubmed:29389600]
Food Res Int. 2018 Jan;103:150-155.
Many mites rely on fungi for nutrients, and fungi benefit from them with regard to spore dispersal, or nutrient resources. The interactions among mites and fungi are still not clear in most cases. This study analyzed volatile natural products from the liquid and solid cultures of the edible fungi, Flammulina velutipes (Fr.) Sing, and the solid mycelia induced by the storage mite, Tyrophagus putrescentiae Schrank, using HS-SPME-GC-MS/MS. Five new monoterpenes and 30 new sesquiterpenes were isolated from the two cultures of F. velutipes and a newly monoterpene and 14 newly sesquiterpenes found in the solid mycelia induced by the storage mite. Sesquiterpenes were abundant in the mycelial stage of F. velutipe. The mite was attracted by some volatiles from host fungi, dihydrocarveol, Cedrol, beta-caryophyllene, alpha-terpilene, beta-pinene and benzaldehyde, analyzed by four-arm olfactometer. Some terpenes induced by T. putrescentiae, such as caryophyllene oxide, bicyclogermacrene, and (-)-spathulenol, would have potential biological function. These results suggest that some volatile sesquiterpenes play an important role in enabling the mite to recognize host fungi.
Cytokine contributions to alterations of the volatile metabolome induced by inflammation.[Pubmed:29241669]
Brain Behav Immun. 2018 Mar;69:312-320.
Several studies demonstrate that inflammation affects body odor. Volatile signals associated with inflammation induced by pyrogens like LPS are detectable both by conspecifics and chemical analyses. However, little is known about the mechanisms which translate detection of a foreign molecule or pathogen into a unique body odor, or even how unique that odor may be. Here, we utilized C57BL/6J trained mice to identify the odor of LPS-treated conspecifics to investigate potential pathways between LPS-induced inflammation and changes in body odor, as represented by changes in urine odor. We hypothesized that the change in volatile metabolites could be caused directly by the pro-inflammatory cytokine response mediated by TNF or IL-1beta, or by the compensatory anti-inflammatory response mediated by IL-10. We found that trained biosensors generalized learned LPS-associated odors to TNF-induced odors, but not to IL-1beta or IL-10-induced odors. Analyses of urine volatiles using headspace gas chromatography revealed distinct profiles of volatile compounds for each treatment. Instrumental discrimination relied on a mixture of compounds, including 2-sec-butyl-4,5-dihydrothiazole, Cedrol, nonanal, benzaldehyde, acetic acid, 2-ethyl-1-hexanol, and dehydro-exo-brevicomin. Although interpretation of LDA modeling differed from behavioral testing, it does suggest that treatment with TNF, IL-1beta, and LPS can be distinguished by their resultant volatile profiles. These findings indicate there is information found in body odors on the presence of specific cytokines. This result is encouraging for the future of disease diagnosis via analysis of volatiles.
The anxiolytic effect of Juniperus virginiana L. essential oil and determination of its active constituents.[Pubmed:29326032]
Physiol Behav. 2018 May 15;189:50-58.
Essential oil from Juniperus virginiana L. (eastern red cedarwood essential oil, CWO) has been used to relax mind and enhance comfort for medical purposes. Few reports showed its effect on anxiety behaviors in animal models. The present study investigated the anxiolytic effect of CWO using two anxiety tests in mice, then determined the major active constituents, examined the change of neurotransmitters after intraperitoneal (i.p.) administration. Analysis using GC/MS revealed that the CWO contained (-)-alpha-cedrene (28.11%), (+)-beta-cedrene (7.81%), (-)-thujopsene (17.71%) and (+)-Cedrol (24.58%). CWO at 400-800mg/kg increased the percentage of open arm entries and the percentage of the time spent in open arms in the elevated plus maze (EPM), suggesting that the oil has anxiolytic effect. However, it didn't show anxiolytic effect in the light-dark box (LDB) test. Tests of the cedrene did not show anxiolytic effect in either test, but rather induced anxiety-related behaviors and inhibited the locomotor activity in EPM and LDB. Cedrol produced significant anxiolytic effect in both EPM and LDB tests at 400-1600mg/kg and 800-1600mg/kg, respectively. A more significant increase in locomotor activity was observed in Cedrol at 200-1600mg/kg administration than CWO. CWO increased the 5-hydroxytryptamine (5-HT) concentration at 800mg/kg, whereas it didn't affect the dopamine (DA) concentration. Cedrol significantly reduced the DA level at 100-200mg/kg and elevated the 5-HT level at 1200-1600mg/kg. Moreover, it changed the ratio of 5-hydroxyindoleacetic acid/5-HT and 3, 4-dihydroxyphenyl acetic acid/DA at 1200-1600mg/kg. CWO and Cedrol, in particular might act in an anxiolytic effect through the 5-HTnergic and DAnergic pathways.


