CassiachromoneCAS# 28955-30-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28955-30-8 | SDF | Download SDF |
| PubChem ID | 5319500 | Appearance | Cryst. |
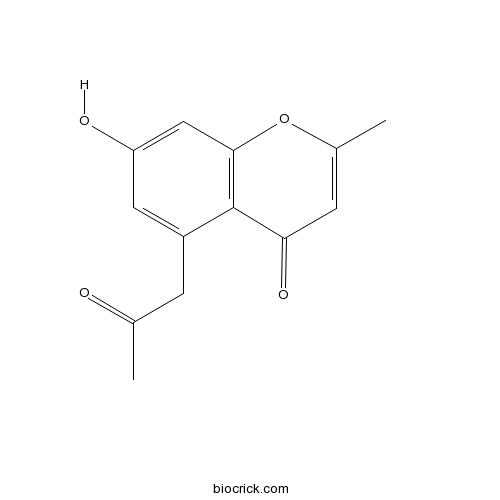
| Formula | C13H12O4 | M.Wt | 232.2 |
| Type of Compound | Chromones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 7-hydroxy-2-methyl-5-(2-oxopropyl)chromen-4-one | ||
| SMILES | CC1=CC(=O)C2=C(O1)C=C(C=C2CC(=O)C)O | ||
| Standard InChIKey | YMCXDPKFRCHLPK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H12O4/c1-7(14)3-9-5-10(15)6-12-13(9)11(16)4-8(2)17-12/h4-6,15H,3H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Targets | Antifection |

Cassiachromone Dilution Calculator

Cassiachromone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3066 mL | 21.5332 mL | 43.0663 mL | 86.1326 mL | 107.6658 mL |
| 5 mM | 0.8613 mL | 4.3066 mL | 8.6133 mL | 17.2265 mL | 21.5332 mL |
| 10 mM | 0.4307 mL | 2.1533 mL | 4.3066 mL | 8.6133 mL | 10.7666 mL |
| 50 mM | 0.0861 mL | 0.4307 mL | 0.8613 mL | 1.7227 mL | 2.1533 mL |
| 100 mM | 0.0431 mL | 0.2153 mL | 0.4307 mL | 0.8613 mL | 1.0767 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Allo-Thr-OH
Catalog No.:BCC3101
CAS No.:28954-12-3
- Canertinib dihydrochloride
Catalog No.:BCC1449
CAS No.:289499-45-2
- Anodendrine
Catalog No.:BCN1956
CAS No.:28942-07-6
- Fmoc-Cl
Catalog No.:BCC2802
CAS No.:28920-43-6
- Triazolam
Catalog No.:BCC5218
CAS No.:28911-01-5
- 3,7,16-Trihydroxystigmast-5-ene
Catalog No.:BCN5191
CAS No.:289056-24-2
- Ergosta-5,24(28)-diene-3,7,16-triol
Catalog No.:BCN5190
CAS No.:289054-34-8
- Z-D-Met-OH
Catalog No.:BCC2758
CAS No.:28862-80-8
- S-(-)-Carbidopa
Catalog No.:BCN8453
CAS No.:28860-95-9
- H-Asp(OBzl)-OBzl.TosOH
Catalog No.:BCC2886
CAS No.:2886-33 -1
- Cediranib (AZD217)
Catalog No.:BCC1121
CAS No.:288383-20-0
- (-)-dicentrine
Catalog No.:BCC8167
CAS No.:28832-07-7
- Oridonin
Catalog No.:BCN5953
CAS No.:28957-04-2
- Lasiodin
Catalog No.:BCN3387
CAS No.:28957-08-6
- Senicapoc
Catalog No.:BCC1943
CAS No.:289656-45-7
- Boc-HomoArg(NO2)-OH
Catalog No.:BCC2646
CAS No.:28968-64-1
- CB 300919
Catalog No.:BCC1456
CAS No.:289715-28-2
- Pectolinarin
Catalog No.:BCN1217
CAS No.:28978-02-1
- Persicoside
Catalog No.:BCN4780
CAS No.:28978-03-2
- Z-N-Me-Phe-OH
Catalog No.:BCC3349
CAS No.:2899-07-2
- H-Trp-Oet.HCl
Catalog No.:BCC2673
CAS No.:2899-28-7
- H-Tryptophanol
Catalog No.:BCC2701
CAS No.:2899-29-8
- H-Methioninol
Catalog No.:BCC2721
CAS No.:2899-37-8
- 3-(Benzylthio)-Propionic acid
Catalog No.:BCC2839
CAS No.:2899-66-3
Stability of barakol under hydrolytic stress conditions and its major degradation product.[Pubmed:19145556]
Planta Med. 2009 Mar;75(4):346-50.
The aim of the present study was to investigate the stability of barakol, an anxiolytic constituent extracted from leaves of Senna siamea (Lam.) Irwin & Barneby (syn. Cassia siamea Lam.), under the International Conference on Harmonisation suggested conditions using HPLC with photodiode array detection. Extensive degradation of barakol was found to occur under alkaline conditions through base-catalyzed hydrolysis. Mild degradation of barakol was observed under thermal and oxidative stress while it was stable under acidic conditions. The reaction rate constants (Kobs) of barakol degradation under alkaline conditions at pHs 12 and 13 were 3.0x10(-5) and 9.6x10(-3) min(-1), respectively. The activation energy according to the Arrhenius plot was calculated to be 26.9+/-3.3 kcal/mol at pH 13 and temperatures between 12 and 51 degrees C. The major degradation product of barakol under both alkaline and thermal stress conditions was characterized by LC-MS and NMR as Cassiachromone.


