CV 1808Non-selective adenosine A2 receptor agonist CAS# 53296-10-9 |
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- Honokiol
Catalog No.:BCN1001
CAS No.:35354-74-6
- Deoxyarbutin
Catalog No.:BCC4774
CAS No.:53936-56-4
- EUK 134
Catalog No.:BCC4317
CAS No.:81065-76-1
- Edaravone
Catalog No.:BCC2480
CAS No.:89-25-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

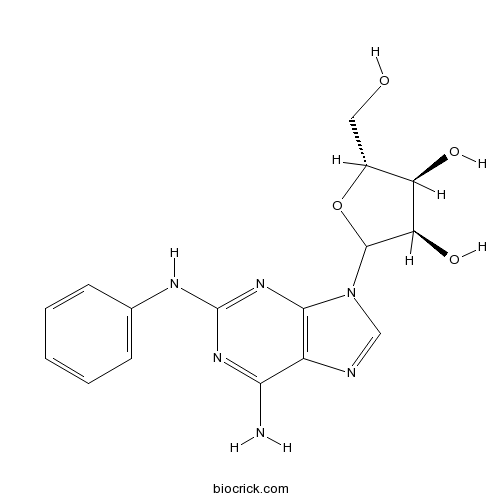
| Cas No. | 53296-10-9 | SDF | Download SDF |
| PubChem ID | 122170 | Appearance | Powder |
| Formula | C16H18N6O4 | M.Wt | 358.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in DMSO | ||
| Chemical Name | (3R,4S,5R)-2-(6-amino-2-anilinopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol | ||
| SMILES | C1=CC=C(C=C1)NC2=NC3=C(C(=N2)N)N=CN3C4C(C(C(O4)CO)O)O | ||
| Standard InChIKey | SCNILGOVBBRMBK-FJFSNTMWSA-N | ||
| Standard InChI | InChI=1S/C16H18N6O4/c17-13-10-14(21-16(20-13)19-8-4-2-1-3-5-8)22(7-18-10)15-12(25)11(24)9(6-23)26-15/h1-5,7,9,11-12,15,23-25H,6H2,(H3,17,19,20,21)/t9-,11-,12-,15?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Adenosine A2 receptor agonist. Coronary vasodilator, antihypertensive and antipsychotic following systemic administration in vivo. |

CV 1808 Dilution Calculator

CV 1808 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7905 mL | 13.9525 mL | 27.9049 mL | 55.8098 mL | 69.7623 mL |
| 5 mM | 0.5581 mL | 2.7905 mL | 5.581 mL | 11.162 mL | 13.9525 mL |
| 10 mM | 0.279 mL | 1.3952 mL | 2.7905 mL | 5.581 mL | 6.9762 mL |
| 50 mM | 0.0558 mL | 0.279 mL | 0.5581 mL | 1.1162 mL | 1.3952 mL |
| 100 mM | 0.0279 mL | 0.1395 mL | 0.279 mL | 0.5581 mL | 0.6976 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- [Ala11,D-Leu15]-Orexin B
Catalog No.:BCC5877
CAS No.:532932-99-3
- Boc-Tyr(Me)-OH
Catalog No.:BCC3268
CAS No.:53267-93-9
- H-Sar-NH2.HCl
Catalog No.:BCC3333
CAS No.:5325-64-4
- 2''-O-Galloylhyperin
Catalog No.:BCN1218
CAS No.:53209-27-1
- Coixol
Catalog No.:BCN5703
CAS No.:532-91-2
- ar-Turmerone
Catalog No.:BCN7516
CAS No.:532-65-0
- Euparin
Catalog No.:BCN7191
CAS No.:532-48-9
- Tropinone
Catalog No.:BCN1935
CAS No.:532-24-1
- Anethole trithione
Catalog No.:BCN8510
CAS No.:532-11-6
- Methyocarbamol
Catalog No.:BCC3813
CAS No.:532-03-6
- Etomidate hydrochloride
Catalog No.:BCC4255
CAS No.:53188-20-8
- Fagomine
Catalog No.:BCC1569
CAS No.:53185-12-9
- Fmoc-Cys(Bzl)-OH
Catalog No.:BCC3475
CAS No.:53298-33-2
- Tigloidine
Catalog No.:BCN1945
CAS No.:533-08-4
- Sesamol
Catalog No.:BCN2594
CAS No.:533-31-3
- 1,2,4-Benzenetriol
Catalog No.:BCC8409
CAS No.:533-73-3
- L-NMMA acetate
Catalog No.:BCC6788
CAS No.:53308-83-1
- Benzyl carbazate
Catalog No.:BCC8872
CAS No.:5331-43-1
- Isogosferol
Catalog No.:BCN5704
CAS No.:53319-52-1
- PP 3
Catalog No.:BCC7486
CAS No.:5334-30-5
- Z-Leu-OH.DCHA
Catalog No.:BCC2765
CAS No.:53363-87-4
- Boc-N-Me-Leu-OH
Catalog No.:BCC2616
CAS No.:53363-89-6
- trans,trans-Bis(4-fluorobenzal)acetone
Catalog No.:BCC9180
CAS No.:53369-00-9
- 1,3,5-Trihydroxy-4-prenylxanthone
Catalog No.:BCN5705
CAS No.:53377-61-0
Inhibition of coronary circulatory failure and thromboxane A2 release during coronary occlusion and reperfusion by 2-phenylaminoadenosine (CV-1808) in anesthetized dogs.[Pubmed:6202970]
J Cardiovasc Pharmacol. 1984 May-Jun;6(3):442-8.
The effects of potent coronary vasodilator, 2- phenylaminoadenosine (CV-1808), on coronary circulatory failure and thromboxane (TX) A2 release were studied during coronary occlusion (for 60 min) and subsequent reperfusion (for 60 min) in anesthetized dogs. During coronary reperfusion, reactive hyperemic response was attenuated, and coronary conductance decreased gradually with time, suggesting coronary circulatory failure. TXA2 release was markedly increased, as demonstrated by contraction of rabbit aortic strips perfused with coronary venous blood draining the ischemic myocardium, and by increased release of radioimmunologically assayable TXB2. CV-1808 (0.25 microgram/kg/min i.v. infusion throughout the experimental period, starting 10 min before coronary occlusion) inhibited coronary circulatory failure and TXA2 release. TXA2 synthetase of horse platelet microsomes was not significantly inhibited (-11.6 +/- 2.1%) by 10(-4) M CV-1808. The compound (10(-5) and 10(-4) M) inhibited collagen-induced TXB2 formation in a dose-dependent manner (-23.0 +/- 9.0 and -74.0 +/- 15.0%, respectively), but not arachidonic acid-induced TXB2 formation by dog platelets, suggesting that CV-1808 inhibited phospholipases. Myocardial infarct size determined 60 min after reperfusion was significantly reduced by CV-1808. Thus, CV-1808 appeared to be effective for salvaging ischemic myocardium. The effect might be related to improvement of coronary circulation and inhibition of release of vasoactive substances, including TXA2, from the ischemic myocardium.
Interaction of 2-phenylaminoadenosine (CV 1808) with adenosine systems in rat tissues.[Pubmed:6295788]
Eur J Pharmacol. 1982 Dec 3;85(3-4):335-8.
2-Chloroadenosine (2-CADO) and 2-phenylaminoadenosine (CV 1808) were compared in a CNS purinergic receptor binding assay and the inhibition of neurogenic contractions of the vas deferens. Both 2-CADO and CV 1808 are more potent than adenosine in both preparations. CV 1808 was 10 times more active than dipyridamole in enhancing the response of the vas deferens to exogenous adenosine. Thus, CV 1808 may owe its potent coronary vasodilator activity to both a direct action on adenosine receptors and the ability to augment adenosine responses.
Adenosine and 2-phenylaminoadenosine (CV-1808) inhibit human neutrophil bactericidal function.[Pubmed:1847698]
Infect Immun. 1991 Mar;59(3):885-9.
Adenosine is a natural autocoid and immunomodulator that serves an anti-inflammatory role. Stimulation of polymorphonuclear neutrophils (PMN) with soluble stimuli has been shown to inhibit the PMN oxidative burst. We examined the effects of adenosine and the adenosine analog 2-phenylaminoadenosine (CV-1808) on PMN bactericidal function. Adenosine (10 mM) and CV-1808 (10 to 100 microM) inhibited PMN killing of Staphylococcus aureus. There were more surviving bacteria after 240 min of incubation of PMN with S. aureus and adenosine (10 mM) or CV-1808 (100 microM) (254% +/- 45% and 739% +/- 88% of control, respectively) (P less than 0.05) than there were in the control. In contrast, inosine (10 mM), the major degradation product of adenosine, did not affect killing. Adenosine and CV-1808 did not alter cell association of S. aureus, but S. aureus-activated PMN superoxide release was decreased by adenosine (10 microM) and CV-1808 (10 microM) to 67% +/- 7% and 32% +/- 12% that of the control, respectively (P less than 0.05). Since adenosine inhibited PMN bactericidal function only at approximately 10,000 times peak physiological concentrations, endogenous adenosine levels would not be expected to adversely affect PMN bactericidal function. On the other hand, pharmacological concentrations of adenosine derivatives may decrease the oxidative burst and killing sufficiently to increase host susceptibility to infection.
[3H]2-phenylaminoadenosine ([3H]CV 1808) labels a novel adenosine receptor in rat brain.[Pubmed:1331404]
J Pharmacol Exp Ther. 1992 Nov;263(2):552-61.
Earlier studies have demonstrated that the vasoactive compound CV 1808 displays 10-fold selectivity for the adenosine A2 receptor, and as such, was the first reported A2-selective agonist. After the radiolabeling of CV 1808, its binding characteristics were evaluated in rat striatal, cortical and hippocampal membranes. Using 5 nM [3H]CV 1808, unlabeled CV 1808 produced shallow inhibition curves in all three brain areas, with 61 to 75% of the binding displaying IC50 values of 16 to 24 nM, whereas the remaining 28 to 37% of binding had lower affinity (IC50 595-1130 nM). The A2-selective agonist CGS 21680 and the nonselective adenosine agonist 5'-N-ethylcarboxamidoadenosine displayed very low affinity (IC50 > 10 microM). The A1-selective compound N6-cyclopentyladenosine inhibited only 28 to 44% of specific binding, with IC50 of 272-1750 nM. In contrast, the nonselective adenosine antagonist CGS 15943A inhibited specific binding by 48 to 64% (at 1 microM) with IC50 ranging from 106 to 295 nM. Additionally, several novel adenosine analogs fully inhibited specific binding, producing multicomponent inhibition curves. Electrophysiological studies in porcine coronary artery cells demonstrated that CV 1808, but not CGS 21680, 5'-N-ethylcarboxamidoadenosine and N6-cyclopentyladenosine, activated potassium channels. Further, the CV 1808-induced activation was blocked by CGS 15943A. These results indicate that [3H]CV 1808 binding consists of two components in rat brain: a low-affinity site with A1-like characteristics, and a novel high-affinity site, designated as the A4 receptor, where potassium channel activation appears to be a functional correlate.
Adenosine agonists reduce conditioned avoidance responding in the rat.[Pubmed:8105493]
Pharmacol Biochem Behav. 1993 Aug;45(4):951-8.
Because adenosine agonists may possess therapeutic potential as antipsychotic agents, we examined the activity of several prototypic agents in vivo in blocking conditioned avoidance responding (CAR) in the rat, a behavioral test predictive of antipsychotic efficacy in humans. Potency in blocking CAR is directly proportional to potency in alleviating schizophrenia. Hence, the adenosine A1-selective agonists [cyclopentyl adenosine (CPA) and (R)-phenylisopropyl adenosine (R-PIA)], A2-selective agonists [CV-1808 and (2-(p-(carboxyethyl)-phenethylamino)-5'-N-ethyl-carboxamido adenosine (CGS 21680)], and a nonselective agonist [5'-N-carboxamido adenosine (NECA)] were examined in this test. Block of CAR was first determined for standard antipsychotic agents [ED50 mg/kg, IP, and 95% confidence level (CL) in parentheses], such as haloperidol [0.23 (0.18, 0.39)], trifluoroperazine [(0.9 (0.7, 1.0)], thioridazine [12.5 (10.5, 15.3)], metoclopramide [7.8 (6.4, 9.2)], and chlorpromazine [4.9 (4.2, 5.9)]. The paradigm consisted of a light- and tone-signaled footshock that could be avoided via a discrete lever press. Affinity for A1 and A2 binding sites in brain tissue from Fischer 344 rats was ascertained to be similar to that seen in other rodent strains. Each adenosine agonist blocked CAR. NECA [ED50 value (95% CL) = 0.07 (0.004, 0.12) mg/kg, IP] was the most potent agent, followed by: R-PIA [0.34 (0.23, 0.44)]; CGS 21680 [1.1 (0.8, 2.0)]; CV-1808 [1.3 (1.0, 1.8)]; and CPA [1.5 (1.3, 1.7)]. Pretreatment with caffeine (25 mg/kg, IP, -10 min) blocked the inhibition of CAR produced by the adenosine agonists, suggesting the event is mediated via purinergic receptors.(ABSTRACT TRUNCATED AT 250 WORDS)
The antihypertensive effect of 2-alkynyladenosines and their selective affinity for adenosine A2 receptors.[Pubmed:1874281]
Eur J Pharmacol. 1991 Apr 10;196(1):69-76.
We examined the affinity for adenosine receptors and the antihypertensive effects of 2-alkynyladenosines, especially 2-hexynyladenosine (2-H-Ado) and 2-octynyladenosine (2-O-Ado). The order of decreasing affinity of 2-H-Ado, 2-O-Ado, and other agonists tested for A1 receptors was N6-cyclopentyladenosine (CPA) greater than N6-cyclohexyladenosine (CHA) greater than N6-R-phenylisopropyladenosine (R-PIA) greater than 2-chloroadenosine (CADO) = 5'-N-ethylcarboxamideadenosine (NECA) greater than N6-S-phenylisopropyladenosine (S-PIA) greater than 2-H-Ado greater than 2-O-Ado greater than 2-phenylaminoadenosine (CV-1808), and that for A2 receptors was 2-H-Ado greater than 2-O-Ado = NECA greater than CADO greater than CV-1808 greater than R-PIA greater than CPA greater than CHA greater than S-PIA. The Ki values of 2-H-Ado and 2-O-Ado for [3H] NECA binding to A2 receptors were 4.1 and 12.1 nM, respectively, and those for [3H]CHA binding to A1 receptors were 146 and 211 nM, respectively: the affinity of 2-H-Ado and 2-O-Ado for A2 receptors was about 36- and 17-fold higher than their affinity for A1 receptors. Injection of 2-H-Ado and 2-O-Ado (0.03-100 micrograms/kg) decreased the blood pressure of anaesthetized spontaneously hypertensive rats (SHR). A slight decrease in heart rate was observed after i.v. injection of 100 micrograms/kg 2-H-Ado and 2-O-Ado. A potent and long-lasting antihypertensive effect was also observed after oral administration of 2-H-Ado and 2-O-Ado to conscious SHR.(ABSTRACT TRUNCATED AT 250 WORDS)


