CFM-2CAS# 178616-26-7 |
- Biperiden HCl
Catalog No.:BCC4565
CAS No.:1235-82-1
- Darifenacin
Catalog No.:BCC1516
CAS No.:133099-04-4
- Darifenacin HBr
Catalog No.:BCC4567
CAS No.:133099-07-7
- Cevimeline hydrochloride hemihydrate
Catalog No.:BCC1471
CAS No.:153504-70-2
- Umeclidinium bromide
Catalog No.:BCC2022
CAS No.:869113-09-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 178616-26-7 | SDF | Download SDF |
| PubChem ID | 4377504 | Appearance | Powder |
| Formula | C17H17N3O3 | M.Wt | 311.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (160.60 mM) *"≥" means soluble, but saturation unknown. | ||
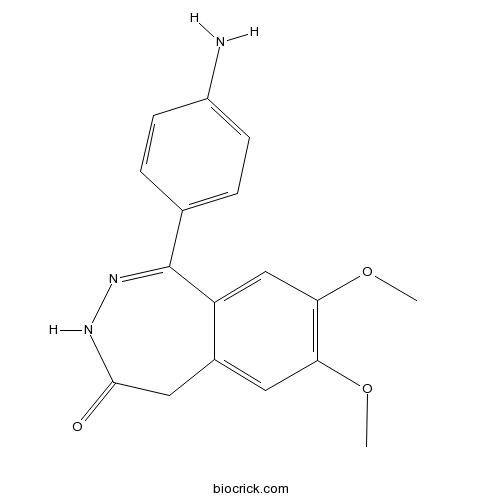
| Chemical Name | 1-(4-aminophenyl)-7,8-dimethoxy-3,5-dihydro-2,3-benzodiazepin-4-one | ||
| SMILES | COC1=C(C=C2C(=C1)CC(=O)NN=C2C3=CC=C(C=C3)N)OC | ||
| Standard InChIKey | MJKADKZSYQWGLL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H17N3O3/c1-22-14-7-11-8-16(21)19-20-17(13(11)9-15(14)23-2)10-3-5-12(18)6-4-10/h3-7,9H,8,18H2,1-2H3,(H,19,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Novel, selective non-competitive AMPA antagonist. Highly potent, long-acting anticonvulsant. |

CFM-2 Dilution Calculator

CFM-2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2119 mL | 16.0596 mL | 32.1192 mL | 64.2385 mL | 80.2981 mL |
| 5 mM | 0.6424 mL | 3.2119 mL | 6.4238 mL | 12.8477 mL | 16.0596 mL |
| 10 mM | 0.3212 mL | 1.606 mL | 3.2119 mL | 6.4238 mL | 8.0298 mL |
| 50 mM | 0.0642 mL | 0.3212 mL | 0.6424 mL | 1.2848 mL | 1.606 mL |
| 100 mM | 0.0321 mL | 0.1606 mL | 0.3212 mL | 0.6424 mL | 0.803 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CFM-2 is a selective non-competitive AMPAR antagonist. IC50 value: Target: AMPAR antagonist in vitro: AMPA antagonists GYKI 52466 and CFM-2 inhibit the extracellular signal regulated kinase (ERK1/2) pathway, CFM-2 reduced phosphorylation of cAMP-responsive element binding protein (CREB), suppressed expression of cyclin D1, upregulated the cell cycle regulators and tumor suppressor proteins p21 and p53 and decreased number of lung adenocarcinoma cells in G2 and S phases of the cell cycle. in vivo: Pretreatment with CFM-2 delayed the progression of seizure rank during repeated administration of pentylentetrazole. At the end of the period of repeated pentylentetrazole treatment (6 weeks) the mean seizure score was 0 in vehicle treated controls, 4.3 in animals treated with vehicle + pentylentetrazole, 2.2 in rats treated chronically with CFM-2 (20 micromol kg(-1) i.p.) + pentylentetrazole and 1.0 in rats treated repeatedly with CFM-2 (50 micromol kg(-1) i.p.) + pentylenetetrazole. CFM-2 was also able to antagonize the long-term increase in sensitivity of the convulsant effects of GABA function inhibitors in pentylentetrazole-kindled animals [1]. CFM-2 has been proven to possess anticonvulsant activity in various models of seizures [2]. Intrathecal application of two selective non-competitive AMPAR antagonists, CFM-2 (25 and 50 microg) and GYKI 52466 (50 microg), significantly attenuated mechanical and thermal hypersensitivities on the ipsilateral hind paw at 2 and 24 h post-CFA injection. Neither CFM-2 nor GYKI 52466 affected the contralateral basal responses to thermal and mechanical stimuli [4].
References:
[1]. De Sarro G, et al. Effects of some AMPA receptor antagonists on the development of tolerance in epilepsy-prone rats and in pentylenetetrazole kindled rats. Eur J Pharmacol. 1999 Mar 5;368(2-3):149-59.
[2]. Rizzo M, et al. Determination of new 2,3-benzodiazepines in rat plasma using high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl. 1999 Aug 20;731(2):207-15.
[3]. Stepulak A, et al. AMPA antagonists inhibit the extracellular signal regulated kinase pathway and suppress lung cancer growth. Cancer Biol Ther. 2007 Dec;6(12):1908-15.
[4]. Park JS, et al. Role of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in complete Freund's adjuvant-induced inflammatory pain. Mol Pain. 2008 Dec 30;4:67.
- U-104
Catalog No.:BCC2312
CAS No.:178606-66-1
- Oleoside
Catalog No.:BCN1134
CAS No.:178600-68-5
- ZD 2079
Catalog No.:BCC5878
CAS No.:178600-17-4
- Prilocaine hydrochloride
Catalog No.:BCC4288
CAS No.:1786-81-8
- Hoechst 33342 analog
Catalog No.:BCC1630
CAS No.:178481-68-0
- Vitexin -4''-O-glucoside
Catalog No.:BCN3054
CAS No.:178468-00-3
- 6-epi-Albrassitriol
Catalog No.:BCN7342
CAS No.:178456-58-1
- Agrostophyllidin
Catalog No.:BCN3598
CAS No.:178439-50-4
- AR-R 17779 hydrochloride
Catalog No.:BCC7827
CAS No.:178419-42-6
- Tos-Arg-OMe.HCl
Catalog No.:BCC2874
CAS No.:1784-03-8
- 12-Hydroxy-6-epi-albrassitriol
Catalog No.:BCN7460
CAS No.:178330-78-4
- H-D-Asp-OH
Catalog No.:BCC2894
CAS No.:1783-96-6
- rel-(8R,8'R)-dimethyl-(7S,7'R)-bis(3,4-methylenedioxyphenyl)tetrahydro-furan
Catalog No.:BCN1521
CAS No.:178740-32-4
- Agrocybenine
Catalog No.:BCN4755
CAS No.:178764-92-6
- Delta3,2-Hydroxylbakuchiol
Catalog No.:BCN3707
CAS No.:178765-49-6
- 3-Hydroxybakuchiol
Catalog No.:BCN3610
CAS No.:178765-54-3
- 12-Hydroxyisobakuchiol
Catalog No.:BCN3609
CAS No.:178765-55-4
- SNC 162
Catalog No.:BCC7103
CAS No.:178803-51-5
- Vitexolide D
Catalog No.:BCN6739
CAS No.:1788090-69-6
- Myricadiol
Catalog No.:BCN1135
CAS No.:17884-88-7
- Salviaflaside
Catalog No.:BCN8330
CAS No.:178895-25-5
- NGD 94-1
Catalog No.:BCC7636
CAS No.:178928-68-2
- 3-(4-Pyridyl)-Alanine
Catalog No.:BCC2651
CAS No.:178933-04-5
- FT-207 (NSC 148958)
Catalog No.:BCC4455
CAS No.:17902-23-7
AMPA Receptor Antagonist CFM-2 Decreases Survivin Expression in Cancer Cells.[Pubmed:29493464]
Anticancer Agents Med Chem. 2018;18(4):591-596.
BACKGROUND: Glutamate receptors are widely expressed in different types of cancer cells. alpha-Amino-3- hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors are ionotropic glutamate receptors which are coupled to intracellular signaling pathways that influence cancer cell survival, proliferation, and migration. Blockade of AMPA receptors by pharmacologic compounds may potentially constitute an effective tool in anticancer treatment strategies. METHOD: Here we investigated the impact of the AMPA receptor antagonist CFM-2 on the expression of the protein survivin, which is known to promote cancer cell survival and proliferation. We show that CFM-2 inhibits survivin expression at mRNA and protein levels and decreases the viability of cancer cells. Using a stably transfected cell line which overexpresses survivin, we demonstrate that over-expression of survivin enhances cancer cell viability and attenuates CFM-2-mediated inhibition of cancer cell growth. RESULT: These findings point towards suppression of survivin expression as a new mechanism contributing to anticancer effects of AMPA antagonists.
Role of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in complete Freund's adjuvant-induced inflammatory pain.[Pubmed:19116032]
Mol Pain. 2008 Dec 30;4:67.
Spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) mediate acute spinal processing of nociceptive and non-nociceptive information, but whether and how their activation contributes to the central sensitization that underlies persistent inflammatory pain are still unclear. Here, we examined the role of spinal AMPARs in the development and maintenance of complete Freund's adjuvant (CFA)-induced persistent inflammatory pain. Intrathecal application of two selective non-competitive AMPAR antagonists, CFM-2 (25 and 50 microg) and GYKI 52466 (50 microg), significantly attenuated mechanical and thermal hypersensitivities on the ipsilateral hind paw at 2 and 24 h post-CFA injection. Neither CFM-2 nor GYKI 52466 affected the contralateral basal responses to thermal and mechanical stimuli. Locomotor activity was not altered in any of the drug-treated animals. CFA-induced inflammation did not change total expression or distribution of AMPAR subunits GluR1 and GluR2 in dorsal horn but did alter their subcellular distribution. The amount of GluR2 was markedly increased in the crude cytosolic fraction and decreased in the crude membrane fraction from the ipsilateral L4-5 dorsal horn at 24 h (but not at 2 h) post-CFA injection. Conversely, the level of GluR1 was significantly decreased in the crude cytosolic fraction and increased in the crude membrane fraction from the ipsilateral L4-5 dorsal horn at 24 h (but not at 2 h) post-CFA injection. These findings suggest that spinal AMPARs might participate in the central spinal mechanism of persistent inflammatory pain.
AMPA antagonists inhibit the extracellular signal regulated kinase pathway and suppress lung cancer growth.[Pubmed:18059166]
Cancer Biol Ther. 2007 Dec;6(12):1908-15. Epub 2007 Sep 1.
Antagonists at alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA)-type glutamate receptors limit growth of human cancers in vitro. However, the mechanism of anticancer action of AMPA antagonists is not known. Here we report that the AMPA antagonists GYKI 52466 and CFM-2 inhibit the extracellular signal regulated kinase (ERK1/2) pathway, an intracellular signaling cascade which is activated by growth factors and controls proliferation of lung adenocarcinoma cells. AMPA antagonists reduced phosphorylation of cAMP-responsive element binding protein (CREB), suppressed expression of cyclin D1, upregulated the cell cycle regulators and tumor suppressor proteins p21 and p53 and decreased number of lung adenocarcinoma cells in G2 and S phases of the cell cycle. These findings reveal potential mechanism of antiproliferative action of AMPA antagonists and indicate that this class of compounds may be useful in the therapy of human cancers.
An alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate glutamate-receptor antagonist can inhibit premicturition contractions in rats with bladder outlet obstruction.[Pubmed:17488306]
BJU Int. 2007 Jul;100(1):181-6.
OBJECTIVE: To explore the possible involvement of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) glutamate-receptors in bladder dysfunction associated with bladder outlet obstruction (BOO), as detrusor overactivity (DO) is common in men with benign prostatic hyperplasia. MATERIALS AND METHODS: Proposed mechanisms of DO include the myogenic or neurogenic theory, and the autonomous bladder hypothesis. In rats, BOO produces premicturition contractions (PMCs) that are assumed to be a consequence of inappropriate non-micturition activity during the filling phase. Using pharmacology, we explored the cause of PMCs to provide new insights into DO in humans. BOO was created in female Wistar rats; 6 weeks after obstruction we evaluated them using conscious-filling cystometry. A specific AMPA receptor antagonist, 1-(4'-aminophenyl)- 3,5-dihydro-7,8-dimethoxy-4H-2,3-benzodiazepin-4-one (CFM-2) was administered intravenously (i.v.) (0.003-3 mg/kg) or intrathecally (i.t.) (0.01-10 microg). RESULTS: The i.v. administration of CFM-2 in rats with BOO significantly decreased the threshold pressure and micturition pressure. The most remarkable findings were that i.v. administration of CFM-2 in rats with BOO significantly and dose-dependently decreased the amplitude and number of PMCs. The highest dose of CFM-2 almost completely eliminated PMCs. The i.t. administration of CFM-2 had no significant effect on PMCs. CONCLUSION: AMPA receptors have never been suggested as a neural mechanism of bladder dysfunction associated with BOO. Although PMCs in rats with BOO have been assumed to be mainly of myogenic origin, PMCs were suppressed by the i.v. administration of CFM-2. Thus, we think that PMCs have neurogenic components that are linked with AMPA receptors. In the present study, i.v. but not i.t. administration of CFM-2 suppressed PMCs, suggesting peripheral and/or supraspinal sites of inhibitory action of CFM-2 on PMCs.
Reduced dendrite growth and altered glutamic acid decarboxylase (GAD) 65- and 67-kDa isoform protein expression from mouse cortical GABAergic neurons following excitotoxic injury in vitro.[Pubmed:17433299]
Exp Neurol. 2007 Jun;205(2):367-82.
The vulnerability of brain cells to neurologic insults varies greatly, depending on their neuronal subpopulation. However, cells surviving pathological insults such as ischemia or brain trauma may undergo structural changes, e.g., altered process growth, that could compromise brain function. In this study, we examined the effect of glutamate excitotoxicity on dendrite growth from surviving cortical GABAergic neurons in vitro. Glutamate exposure did not affect GABAergic neuron viability, however, it significantly reduced dendrite growth from GABAergic neurons. This effect was blocked by the AMPA receptor antagonists NBQX and CFM-2, and mimicked by AMPA, but not NMDA. Glutamate excitotoxicity also caused an NMDA receptor-mediated decrease in the GABA synthesizing enzyme glutamic acid decarboxylase (GAD65/67) immunoreactivity from GABAergic neurons, measured using immunocytochemical and Western blot techniques. GAD is necessary for GABA synthesis; however, reduction of GABA by 3-mercaptopropionic acid (3-MPA), which inhibits GABA synthesis, did not alter dendrite growth. These results suggest that GABAergic cortical neurons are relatively resistant to excitotoxic-induced cell death, but they can display morphological and biochemical alterations which may impair their function.
Effects of non-competitive AMPA receptor antagonists injected into some brain areas of WAG/Rij rats, an animal model of generalized absence epilepsy.[Pubmed:16901515]
Neuropharmacology. 2006 Nov;51(6):1058-67.
CFM-2 [1-(4-aminophenyl)-3,5-dihydro-7,8-dimethoxy-4H-2,3-benzodiazepin-4-one] and THIQ-10c [N-acetyl-1-(4-chlorophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline], are two non-competitive 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl) propionic acid (AMPA) receptor antagonists, which demonstrated to antagonize generalized tonic-clonic seizures in different animal models. We have evaluated the effects of such compounds in a genetic animal model of absence epilepsy, the WAG/Rij rat. Animals were focally microinjected into specific brain areas of the cortico-thalamic circuit in order to evaluate the effects of these compounds on the number and duration of epileptic spike-wave discharges (SWDs) and better characterize the role of AMPA neurotransmission in this animal model. The focal microinjection of the two AMPA antagonists into some thalamic nuclei (ventralis posteromedialis (VPM), reticularis (NRT), ventralis posterolateralis (VPL) and the primary somatosensory forelimb region (S1FL)) was, generally, not able to significantly modify the occurrence of SWDs. Whereas, both compounds were able to reduce the number and duration of SWDs dose-dependently when microinjected into the peri-oral region of the primary somatosensory cortex (S1po). These findings suggest that AMPA receptor antagonists might play a role in absence epilepsies and that it might depend on the involvement of specific neuronal areas.
Effect of glutamate receptor antagonists and antirheumatic drugs on proliferation of synoviocytes in vitro.[Pubmed:16533508]
Eur J Pharmacol. 2006 Mar 27;535(1-3):95-7.
One of the most striking features of inflammatory arthritis is the hyperplasia of synovial fibroblasts. It is not known whether the massive synovial hyperplasia characteristic of rheumatoid arthritis is due to the proliferation of synovial fibroblasts or to defective apoptosis. It has been found that glutamate receptor antagonists inhibit proliferation of different human tumour cells and the anticancer potential of glutamate receptor antagonists was suggested. Here, we investigated the effect of glutamate receptor antagonists and selected antirheumatic drugs on proliferation of synoviocytes in vitro. Experiments were conducted on rabbit synoviocytes cell line HIG-82 obtained from American Type Culture Collection (Menassas, VA, USA). Cell proliferation was assessed by means of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The IC50 value (the concentration of drug necessary to induce 50% inhibition) together with confidence limits was calculated. Glutamate receptor antagonists, 1-(4-aminophenyl)-3,5-dihydro-7,8-dimethoxy-4H-2,3-benzodiazepin-4-one (CFM-2), riluzole, memantine, 1-4-aminophenyl-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine (GYKI 52466), dizocilpine, ketamine and 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline (NBQX), inhibited proliferation of synoviocytes with the following IC50 values (in mM): 0.014, 0.017, 0.065, 0.102, 0.15, 0.435 and 1.16, respectively. Antirheumatic drugs, celecoxib, diclofenac, nimesulide, sulfasalazine, naproxen and methotrexate, inhibited proliferation of synoviocytes with the following IC50 values (in mM): 0.0043, 0.034, 0.044, 0.096, 0.385 and 1.123, respectively. Thus, the antiproliferative potential of glutamate receptor antagonists is comparable to that of antirheumatic drugs.
Glutamate receptor agonist kainate enhances primary dendrite number and length from immature mouse cortical neurons in vitro.[Pubmed:16498632]
J Neurosci Res. 2006 May 1;83(6):944-56.
Glutamate is an important regulator of dendrite development that may inhibit, (during ischemic injury), or facilitate (during early development) dendrite growth. Previous studies have reported mainly on the N-methyl-D-aspartate (NMDA) receptor-mediated dendrite growth-promoting effect of glutamate. In this study, we examined how the non-NMDA receptor agonist kainate influenced dendrite growth. E18 mouse cortical neurons were grown for 3 days in vitro and immunolabeled with anti-microtubule-associated protein 2 (MAP2) and anti-neurofilament (NF-H), to identify dendrites and axons, respectively. Exposure of cortical neurons to kainate increased dendrite growth without affecting neuron survival. This effect was dose-dependent, reversible and blocked by the alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionate (AMPA)/kainate receptor antagonist NBQX and the low-affinity kainate receptor antagonist NS-102, but not by the AMPA receptor antagonist CFM-2. In addition, the NMDA receptor antagonist MK-801 had no effect on kainate-induced dendrite growth. Immunolabeling and Western blot analysis of kainate receptors using antibodies against the GluR6 and KA2 subunits, demonstrated that the immature cortical neurons used in this study express kainate receptor proteins. These results suggest that kainate-induced non-NMDA receptor activation promotes dendrite growth, and in particular primary dendrite number and length, from immature cortical neurons in vitro, and that kainate receptors may be directly involved in this process. Furthermore, these data support the possibility that like NMDA receptors, kainate receptor activation may also contribute to early neurite growth from cortical neurons in vitro.
Glutamate receptors on human melanocytes regulate the expression of MiTF.[Pubmed:16420247]
Pigment Cell Res. 2006 Feb;19(1):58-67.
Glutamate is the major excitatory neurotransmitter in the central nervous system but has also important functions in the epidermis. It is involved in keratinocyte barrier function and in re-epithelialization processes after wounding. Recently, glutamate signalling has been suggested to be implicated in the development of melanoma. The present study examined the expression and functionality of metabotropic and ionotropic glutamate receptors on normal human melanocytes. We found that cultured melanocytes expressed the ionotropic glutamate receptors GluR2 and 4 [alpha-amino-3-hydroxy-5-methyl-4-isoxsazolepropionic acid (AMPA) receptors] and N-methyl-d-aspartate (NMDA) receptors 2A and 2C and possibly the metabotropic glutamate receptor 1. Melanocytes were also found to express specific glutamate transporters and decarboxylases, but appeared neither to produce nor to release l-glutamate. Stimulation with 10 or 100 microM AMPA or NMDA elevated intracellular calcium concentrations in melanocytes, and thus demonstrated the functionality of the glutamate receptors. Millimolar concentrations of l-glutamate did not induce melanocyte toxicity and had no stimulating effect on melanin production. However, blockage of AMPA and NMDA receptors with CFM-2, memantine or MK801 caused a rapid and reversible change in melanocyte morphology, which was associated with disorganisation of actin and tubulin microfilaments. After 24 h of treatment with the AMPA receptor inhibitor CFM-2, there was a sharp reduction in the expression of the crucial melanocyte differentiation and proliferation factor MiTF. The results of this study demonstrate a role for glutamate in melanocyte regulation that may have implications in melanocyte associated disorders.
Group III mGlu receptor agonists potentiate the anticonvulsant effect of AMPA and NMDA receptor block.[Pubmed:12223229]
Eur J Pharmacol. 2002 Sep 6;451(1):55-61.
We report the anticonvulsant action in DBA/2 mice of two mGlu Group III receptor agonists: (R,S)-4-phosphonophenylglycine, (R,S)-PPG, a compound with moderate mGlu8 selectivity, and of (1S,3R,4S)-1-aminocyclopentane-1,2,4-tricarboxylic acid, ACPT-1, a selective agonist for mGlu4alpha receptors. Both compounds, given intracerebroventricularly at doses which did not show marked anticonvulsant activity, produced a consistent shift to the left of the dose-response curves (i.e. enhanced the anticonvulsant properties) of 1-(4'-aminophenyl)-3,5-dihydro-7,8-dimethoxy-4H-2,3-benzodiazepin-4-one hydrochloride, CFM-2, a noncompetitive AMPA receptor antagonist, and 3-((+/-)-2-carboxypiperazin-4-yl)-1-phosphonic acid, CPPene, a competitive NMDA receptor antagonist, in DBA/2 mice. In addition, (R,S)-PPG and ACPT-1 administered intracerebroventricularly prolonged the time course of the anticonvulsant properties of CFM-2 (33 micromol/kg, i.p.) and CPPene (3.3 micromol/kg, i.p.) administered intraperitoneally. We conclude that modest reduction of synaptic glutamate release by activation of Group III metabotropic receptors potentiates the anticonvulsant effect of AMPA and NMDA receptor blockade.
Determination of new 2,3-benzodiazepines in rat plasma using high-performance liquid chromatography with ultraviolet detection.[Pubmed:10510773]
J Chromatogr B Biomed Sci Appl. 1999 Aug 20;731(2):207-15.
A method for the analysis of [1-(4-aminophenyl)-3,5-dihydro-7, 8-dimethoxy-4H-2,3-benzodiazepin-4-one] (CFM-2) and its analogues CFM-3, CFM-4 and CFM-5 in rat plasma was developed. The 2,3-benzodiazepines (2,3-BZs) were extracted by liquid-liquid extraction and analyzed using high-performance liquid chromatography (HPLC) with ultraviolet detection (UV) at 240 nm. The method exhibited a large linear range from 0.05 to 2 micrograms/ml with an intra-assay accuracy for all studied compounds ranging from 92 to 105.5%; whereas the intra-assay precision ranged from 0.59 to 8.16% in rat plasma. The inter-assay accuracy of CFM-2, CFM-4 and their 3-methyl derivatives, CFM-3 and CFM-5 ranged from 92.2 to 107% and the inter-assay precision ranged from 2.17 to 11.9% in rat plasma. The lower limit of detection was 5.5 ng/ml for CFM-2, 6.5 ng/ml for CFM-3, 7 ng/ml for CFM-4 and 8.5 ng/ml for CFM-5 in rat plasma. The pharmacokinetic study demonstrated that 2,3-BZs achieved a peak plasma concentration between 45 and 75 min after drug administration. Moreover, we observed that plasma chromatograms of rats treated with CFM-3, CFM-4 and CFM-5, respectively, showed a peak consistent with CFM-2. Our study suggests that CFM-4, CFM-5 and CFM-3 are prodrugs of CFM-2, in which they are biotransformed in vivo via different metabolic pathways. In particular, CFM-2 has been proven to possess anticonvulsant activity in various models of seizures, acting as alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionate (AMPA) receptor antagonist.
Anticonvulsant activity and plasma level of 2,3-benzodiazepin-4-ones (CFMs) in genetically epilepsy-prone rats.[Pubmed:10462191]
Pharmacol Biochem Behav. 1999 Aug;63(4):621-7.
Anticonvulsant properties of some 2,3-benzodiazepine derivatives acting as alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA) antagonists have been examined in vivo in the genetically epilepsy-prone rats using an audiogenic seizures assay. 2,3-Benzodiazepin-4-ones (CFMs) are nonselective AMPA antagonists that have been found to be potent anticonvulsant compound is in acute models of epilepsy. Because very little is known about their actions in a chronic model of epilepsy, and no correlations exist between anticonvulsant potency and plasma levels of these derivatives, we planned to investigate such a relationship. Maximal anticonvulsant protection occurred 15-60 min after the IP administration of GYKI 52466, 30-90 min after CFM-2, and 45-120 min after CFM-3. In addition, maximal anticonvulsant effect was observed 60-120 min after the IP administration of CFM-4 and at 90 min after CFM-5. The therapeutic index revealed that GYKI 52466 was slightly more toxic than CFM-2 and CFM-3. The time course of plasma levels of rats treated showed that peak plasma concentration was observed 45 min after IP administration of CFM-2 and CFM-3 and 75 min after CFM-4 and CFM-5. Following IP administration of CFM-3 two curves were detected, one is referred to the injected compound, and the other to its demethylated metabolite, which corresponds to CFM-2. Also. for the nitroderivative CFM-4 two curves were detected: one of an injected compound and the second due to its reduced metabolite (CFM-2). Finally, three different metabolites were detected in rat plasma after IP administration of CFM-5. The present study demonstrated that CFMs showed a significant protection against auditory stimulation during the period of peak plasma concentrations, suggesting a marked inhibition of those brain structures involved in the initiation and/or spreading of the audiogenic seizures.
Effects of some AMPA receptor antagonists on the development of tolerance in epilepsy-prone rats and in pentylenetetrazole kindled rats.[Pubmed:10193651]
Eur J Pharmacol. 1999 Mar 5;368(2-3):149-59.
The non-selective alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA) receptor antagonists, 2,3-benzodiazepine derivatives CFM-1 (3,5-dihydro-7,8-dimethoxy-1-phenyl-4H-2,3-benzodiazepin-4-one) and CFM-2 (1-(4'-aminophenyl)-3,5-dihydro-7,8-dimethoxy-4H-2,3-benzodiazepin -4-one), following intraperitoneal (i.p.) administration, were studied against audiogenic seizures in genetically epilepsy-prone rats (GEPRs) or pentylenetetrazole induced kindling in rats. After acute i.p. administration the ED50 values of CFM-1 against the clonic and tonic phases of the audiogenic seizures 30 min after pretreatment were 40 (16-100) and 13 (8-25) micromol kg(-1), respectively. The animals used for chronic study were treated i.p. daily (at 10 h) for 4 weeks with CFM-1 (20 or 50 micromol kg(-1)). Chronic treatment for 2 weeks with CFM-1 gave ED50 values against clonic and tonic seizures of 39 (22-69) and 16 (8-25) micromol kg(-1), respectively, whereas chronic treatment for 4 weeks gave ED50 values against clonic and tonic seizures of 42 (18-98) and 17 (7-41.3) micromol kg(-1), respectively. The duration of anticonvulsant activity observed between 0.5 and 4 h following administration of CFM-1 was similar for acute and chronic treatment. Two groups of Sprague-Dawley rats received CMF (20 or 50 micromol kg(-1)) 30 min before a subconvulsant dose of pentylentetrazole (25 mg kg(-1) i.p.) which is able to increase seizure severity in control animals (i.e., chemical kindling). Pretreatment with CFM-2 delayed the progression of seizure rank during repeated administration of pentylentetrazole. At the end of the period of repeated pentylentetrazole treatment (6 weeks) the mean seizure score was 0 in vehicle treated controls, 4.3 in animals treated with vehicle + pentylentetrazole, 2.2 in rats treated chronically with CFM-2 (20 micromol kg(-1) i.p.) + pentylentetrazole and 1.0 in rats treated repeatedly with CFM-2 (50 micromol kg(-1) i.p.) + pentylenetetrazole. CFM-2 was also able to antagonize the long-term increase in sensitivity of the convulsant effects of GABA function inhibitors in pentylentetrazole-kindled animals. Thus, the administration of a challenge dose of pentylentetrazole (15 mg kg(-1) i.p.) or picrotoxin (1.5 mg kg(-1) i.p.) 15 or 30 days after the end of the repeated treatment showed that animals treated with CFM-2 were significantly protected against seizures induced by pentylentetrazole or picrotoxin. The data suggest that, following repeated treatment, tolerance to the novel AMPA receptor antagonists does not develop (CFM-1 in genetically epilepsy-prone rats and CFM-2 in the pentylentetrazole kindling model of epilepsy). Thirteen minutes after drug injection on days 1, 14 and 28 of chronic treatment the motor impairment induced by these compounds was studied with a rotarod apparatus. The TD50 values for CFM-1 or CFM-2-induced impairment of locomotor performance were similar following acute and repeated treatment. The data also suggest that some novel 2,3-benzodiazepines may have clinical potential for some types of epilepsy.
1-Aryl-3,5-dihydro-4H-2,3-benzodiazepin-4-ones: novel AMPA receptor antagonists.[Pubmed:9111300]
J Med Chem. 1997 Apr 11;40(8):1258-69.
Our previous publication (Eur. J. Pharmacol. 1995, 294, 411-422) reported preliminary chemical and biological studies of some 2,3-benzodiazepines, analogues of 1-(4-aminophenyl)-4-methyl-7,8-(methylenedioxy)-5H-2,3-benzodiazepine (1, GYKI 52466), which have been shown to possess significant anticonvulsant activity. This paper describes the synthesis of new 1-aryl-3,5-dihydro-4H-2,3-benzodiazepin-4-ones and the evaluation of their anticonvulsant effects. The observed findings extend the structure-activity relationships previously suggested for this class of anticonvulsants. The seizures were evoked both by means of auditory stimulation in DBA/2 mice and by pentylenetetrazole or maximal electroshock in Swiss mice. 1-(4'-Aminophenyl)- (38) and 1-(3'-aminophenyl)-3,5-dihydro-7,8-dimethoxy-4H-2,3-benzodiazepin- 4-one (39), the most active compounds of the series, proved to be more potent than 1 in all tests employed. In particular, the ED50 values against tonus evoked by auditory stimulation were 12.6 micromol/kg for derivative 38, 18.3 micromol/kg for 39, and 25.3 micromol/kg for 1. Higher doses were necessary to block tonic extension induced both by maximal electroshock and by pentylenetetrazole. In addition these compounds exhibited anticonvulsant properties that were longer lasting than those of compound 1 and were less toxic. The novel 2,3-benzodiazepines were also investigated for a possible correlation between their anticonvulsant activities against convulsions induced by 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionic acid (AMPA) and their affinities for benzodiazepine receptors (BZR). The 2,3-benzodiazepines did not affect the binding of [3H]flumazenil to BZR, and conversely, their anticonvulsant effects were not reversed by flumazenil. On the other hand the 2,3-benzodiazepines antagonized seizures induced by AMPA and aniracetam in agreement with an involvement of the AMPA receptor. In addition, both the derivative 38 and the compound 1 markedly reduced the AMPA receptor-mediated membrane currents in guinea-pig olfactory cortical neurons in vitro in a noncompetitive manner. The derivatives 25 and 38-40 failed to displace specific ligands from N-methyl-D-aspartate (NMDA), AMPA/kainate, or metabotropic glutamate receptors.


