ByakangelicinCAS# 482-25-7 |
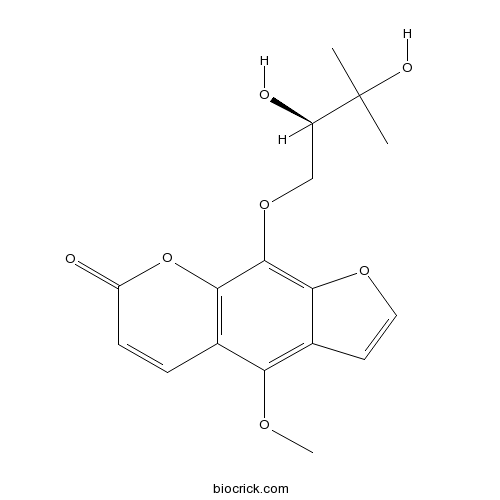
- (+-)-Byakangelicin
Catalog No.:BCN5000
CAS No.:19573-01-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 482-25-7 | SDF | Download SDF |
| PubChem ID | 10211 | Appearance | White powder |
| Formula | C17H18O7 | M.Wt | 334.32 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Synonyms | Biacangelicin; Bjacangelicin | ||
| Solubility | Soluble in DMSO, ethanol and methan | ||
| Chemical Name | 9-[(2R)-2,3-dihydroxy-3-methylbutoxy]-4-methoxyfuro[3,2-g]chromen-7-one | ||
| SMILES | CC(C)(C(COC1=C2C(=C(C3=C1OC(=O)C=C3)OC)C=CO2)O)O | ||
| Standard InChIKey | PKRPFNXROFUNDE-LLVKDONJSA-N | ||
| Standard InChI | InChI=1S/C17H18O7/c1-17(2,20)11(18)8-23-16-14-10(6-7-22-14)13(21-3)9-4-5-12(19)24-15(9)16/h4-7,11,18,20H,8H2,1-3H3/t11-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Byakangelicin Dilution Calculator

Byakangelicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9911 mL | 14.9557 mL | 29.9115 mL | 59.8229 mL | 74.7787 mL |
| 5 mM | 0.5982 mL | 2.9911 mL | 5.9823 mL | 11.9646 mL | 14.9557 mL |
| 10 mM | 0.2991 mL | 1.4956 mL | 2.9911 mL | 5.9823 mL | 7.4779 mL |
| 50 mM | 0.0598 mL | 0.2991 mL | 0.5982 mL | 1.1965 mL | 1.4956 mL |
| 100 mM | 0.0299 mL | 0.1496 mL | 0.2991 mL | 0.5982 mL | 0.7478 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neolancerin
Catalog No.:BCN9027
CAS No.:117221-65-5
- (-)-Carvone
Catalog No.:BCN8949
CAS No.:6485-40-1
- Cyanidin-3-O-(6''-malonylglucoside) chloride
Catalog No.:BCN9026
CAS No.:171828-62-9
- Sipeimine-3-beta-D-glucoside
Catalog No.:BCN9025
CAS No.:67968-40-5
- Robinetinidin chloride
Catalog No.:BCN9024
CAS No.:3020-09-5
- (+)-Mediresinol Di-O-beta-D-glucopyranoside
Catalog No.:BCN9023
CAS No.:88142-63-6
- Peonidin-3,5-O-diglucoside chloride
Catalog No.:BCN9022
CAS No.:132-37-6
- Guibourtinidin chloride
Catalog No.:BCN9021
CAS No.:23130-31-6
- Regaloside A
Catalog No.:BCN9020
CAS No.:114420-66-5
- Regaloside B
Catalog No.:BCN9019
CAS No.:114420-67-6
- Isofutoquinol A
Catalog No.:BCN9018
CAS No.:62499-70-1
- Delphinidin-3-O-sambubioside-5-O-glucoside chloride
Catalog No.:BCN9017
CAS No.:36415-91-5
- Cynatratoside C
Catalog No.:BCN9029
CAS No.:
- (±)-Nicotine
Catalog No.:BCN9030
CAS No.:22083-74-5
- Galactaric acid
Catalog No.:BCN9031
CAS No.:526-99-8
- Anethole, trans-
Catalog No.:BCN9032
CAS No.:4180-23-8
- Cichoric acid
Catalog No.:BCN9033
CAS No.:6537-80-0
- Isopongachromene
Catalog No.:BCN9034
CAS No.:
- 1-Naphthaleneacetic acid
Catalog No.:BCN9035
CAS No.:86-87-3
- Kizuta saponin K11
Catalog No.:BCN9036
CAS No.:97240-03-4
- D-(+)-Lactose Monohydrate
Catalog No.:BCN9037
CAS No.:64044-51-5
- Ganoderic acid E
Catalog No.:BCN9038
CAS No.:
- Fluoranthene
Catalog No.:BCN9039
CAS No.:206-44-0
- DL-α-Tocopherol
Catalog No.:BCN9040
CAS No.:10191-41-0
Byakangelicin protects against carbon tetrachloride-induced liver injury and fibrosis in mice.[Pubmed:32643868]
J Cell Mol Med. 2020 Jul 9.
Liver fibrosis is a disease caused by long-term damage that is related to a number of factors. The current research on the treatment of liver fibrosis mainly focuses on the activation of hepatic stellate cell, in addition to protecting liver cells. Byakangelicin has certain anti-inflammatory ability, but its effect on liver fibrosis is unclear. This study aims to explore whether Byakangelicin plays a role in the development of liver fibrosis and to explore its mechanism. We determined that Byakangelicin has a certain ability to resist fibrosis and reduce liver cell damage in a model of carbon tetrachloride-induced liver fibrosis in mice. Thereafter, we performed further verification in vitro. The signalling pathways of two important pro-fibrotic cytokines, transforming growth factor-beta and platelet-derived growth factor, were studied. Results showed that Byakangelicin can inhibit related pathways. According to the hepatoprotective effect of Byakangelicin observed in animal experiments, we studied the effect of Byakangelicin on 4-HNE-induced hepatocyte (HepG2) apoptosis and explored its related pathways. The results showed that Byakangelicin could attenuate 4-HNE-induced hepatocyte apoptosis via inhibiting ASK-1/JNK signalling. In conclusion, Byakangelicin could improve carbon tetrachloride-induced liver fibrosis and liver injury by inhibiting hepatic stellate cell proliferation and activation and suppressing hepatocyte apoptosis.
Byakangelicin inhibits IL-1beta-induced mouse chondrocyte inflammation in vitro and ameliorates murine osteoarthritis in vivo.[Pubmed:32485353]
Int Immunopharmacol. 2020 Aug;85:106605.
Osteoarthritis (OA) is a chronic musculoskeletal degeneration disease, resulting in severe consequences such as chronic pain and functional disability. Owing to the complex pathology, there are currently available preventative clinical therapies for OA. Several studies have reported the potential anti-inflammatory effects of Byakangelicin (BYA), a component of the Angelica dahurica root extract; however, the effects of BYA in OA remain unknown. In this study, we investigated the protective effects of BYA in interleukin (IL)-1beta-induced mouse chondrocytes in vitro and on surgical destabilization in a medial meniscus (DMM) mouse OA model in vivo. In vitro, BYA treatment inhibited IL-1beta-mediated inducible nitric oxide synthase, cyclooxygenase-2, tumor necrosis factor-alpha, and IL-6 expression. Moreover, BYA promoted the expression of type two collagen and aggrecan but inhibited the expression of thrombospondin motifs 5 and matrix metalloproteinases, leading to degradation of the extracellular matrix. In addition, BYA mechanistically suppressed nuclear factor-kappa B signaling in the IL-1beta-induced chondrocytes. The protective effects of BYA in OA development were also observed in vivo using the DMM model. In conclusion, our results highlight BYA as a candidate for OA treatment and prevention.
Simultaneous Determination of Three Coumarins in Angelica dahurica by (1)H-qNMR Method: A Fast and Validated Method for Crude Drug Quality Control.[Pubmed:32280555]
J Anal Methods Chem. 2020 Mar 24;2020:8987560.
In this study, a quantitative (1)H NMR method ((1)H-qNMR) for determining the contents of imperatorin, Byakangelicin, and oxypeucedanin in A. dahurica in traditional Chinese medicine (TCM) has been established. Dried plant material was extracted exhaustively with methanol by an ultrasonication-assisted extraction method. The (1)H-qNMR measurements were performed on a 600 -MHz spectrometer with hydroquinone as the internal standard reference in deuterated dimethyl sulfoxide (DMSO-d6) solvent. Quantification was carried out using the (1)H resonance signals at 6.55 ppm for hydroquinone and 7.68, 7.38-7.39, and 6.38-6.39 ppm for imperatorin, Byakangelicin, and oxypeucedanin, respectively. The linearity, limit of detection (LOD), limit of quantitation (LOQ), precision, reproducibility, stability, and recovery of the methodology were evaluated, and results were good. The newly developed method has been applied to determine the three coumarins in A. dahurica.
Effect of co-administration of Acori Tatarinowii Rhizoma volatile oil on pharmacokinetic fate of xanthotoxol, oxypeucedanin hydrate, and byakangelicin from Angelicae Dahuricae Radix in rat.[Pubmed:32222035]
J Sep Sci. 2020 Jun;43(12):2349-2362.
A combination of Angelicae Dahuricae Radix and Acori Tatarinowii Rhizoma has been widely used as the herb pair in traditional Chinese medicine to treat stroke, migraine, and epilepsy. However, the underlying synergistic mechanism of the herb pair remains unknown. This study was aimed at investigating the effects of Acori Tatarinowii Rhizoma volatile oil on the pharmacokinetic parameters of xanthotoxol, oxypeucedanin hydrate, and Byakangelicin from Angelicae Dahuricae Radix in rat, and in vitro absorption behavior of the three compounds using rat everted gut sac, in situ single-pass intestinal perfusion, and Caco-2 cell monolayer models. The pharmacokinetic study exhibited clear changes in the key pharmacokinetic parameters of the three main coumarins through co-administering with Acori Tatarinowii Rhizoma volatile oil (50 mg/kg), the area under curve and the maximum plasma concentration of xanthotoxol increased 1.36 and 1.31 times; the area under curve, the maximum plasma concentration, mean residence time, half-life of elimination, and the time to reach peak concentration of oxypeucedanin hydrate increased by 1.35, 1.18, 1.24, 1.19 and 1.49 times, respectively; the area under curve, mean residence time, half-life of elimination, and time to reach peak concentration of Byakangelicin climbed 1.29, 1.27, 1.37, and 1.28 times, respectively. The three coumarin components were absorbed well in the jejunum and ileum in the intestinal perfusion model, when co-administered with Acori Tatarinowii Rhizoma volatile oil (100 mug/mL). The in vivo and in vitro experiments showed good relevance and consistency. The results demonstrated that the three coumarin compounds from Angelicae Dahuricae Radix were absorbed through the active transportation, and Acori Tatarinowii Rhizoma volatile oil could promote the intestinal absorption and transport of these compounds by inhibiting P-glycoprotein (P-gp)-mediated efflux.
Tissue distribution study of Angelica dahurica cv. Yubaizhi in rat by ultra-performance liquid chromatography with tandem mass spectrometry.[Pubmed:31153136]
J Pharm Biomed Anal. 2019 Sep 10;174:43-49.
A sensitive and specific ultra-performance liquid chromatographic-tandem mass (UPLC-MS/MS) spectrometric method was established to investigate tissue distribution of fourteen coumarins of Angelica Dahurica cv. Yubaizhi roots (ADYR) in rat tissues, including isoimperatorin (1), imperatorin (2), isooxypeucedanin (3), Byakangelicin (4), oxypeucedanin hydrate (5), bergapten (6), 2"R-neobyakangelicol (7), phellopterin (8), xanthotoxin (9), isopimpinellin (10), oxypeucedanin ethanolate (11), isobyakangelicol (12), columbianetin (13), (-)-marmesin (14). Detection was performed on a triple quadrupole mass spectrometer in multiple-reaction-mode (MRM). The method established in this assay was successfully applied to tissue distribution study of the selected 14 coumarins after oral administration of the extract of ADYR in rat tissues, including heart, liver, spleen, lung, kidney, stomach, small intestine, muscle, testis, and brain. Tissue distribution characteristics of the fourteen coumarins were clearly elucidated, and the results of this study indicated that the fourteen coumarins were distributed to rat tissues rapidly and could be detected in all of the selected tissues after oral administration. Concentrations of the coumarins were obviously higher in kidney, liver and stomach tissues, and lower in testis, brain and muscle tissues. As an important part of ADMET/Act. study on ADYR, the tissue distribution of multiple coumarins of ADYR in rats provides a significant basis for better evaluation of the metabolism and disposition process in vivo of the herb medicine. The information provided in this research is very useful for further understanding of the metabolic mechanism of ADYR in vivo.
Byakangelicin as a modulator for improved distribution and bioactivity of natural compounds and synthetic drugs in the brain.[Pubmed:31128487]
Phytomedicine. 2019 Sep;62:152963.
BACKGROUND: The elucidation of the biological roles of individual active compounds in terms of their in vivo bio-distribution and bioactivity could provide crucial information to understand how natural compounds work together as treatments for diseases. PURPOSE: We examined the functional roles of Byakangelicin (Byn) to improve the brain accumulation of active compounds, e.g., umbelliferone (Umb), curcumin (Cur), and doxorubicin (Dox), and consequently to enhance their biological activities. METHODS: Active compounds were administered intravenously to mice, with or without Byn, after which organs were isolated and visualized for their ex vivo fluorescence imaging to determine the bio-distribution of each active compound in vivo. For the in vivo bioactivity, Cur, either with or without Byn, was administered to a lipopolysaccharide (LPS)-induced neuro-inflammation model for 5 days, and its anti-inflammatory effects were examined by ELISA using a brain homogenate and serum. RESULTS: We successfully demonstrated that the levels of active compounds (Umb, Cur, and Dox) in the brain, lung, and pancreas were greatly elevated by the addition of Byn via direct ex vivo fluorescence monitoring. In addition, sufficient accumulation of the active compound, Cur, greatly reduced LPS-induced neuro-inflammation in vivo. CONCLUSION: Byn could serve as a modulator to allow improved brain accumulation of diverse active compounds (Umb, Cur, and Dox) and enhanced therapeutic effects.
Enhanced intracellular uptake and stability of umbelliferone in compound mixtures from Angelica gigas in vitro.[Pubmed:31105023]
J Pharmacol Sci. 2019 May;140(1):8-13.
Understanding how natural compounds work together for disease treatments can improve their clinical efficacy and therapeutic effects. To elucidate the mechanisms of synergistic biological effects in natural compound mixtures, umbelliferone (UMB, 7-hydroxycoumarin), derived from Angelica (A.) gigas, was selected as active compound with fluorescent characteristic to examine bioactivities in vitro in the presence of other compounds from Angelica gigas. Antioxidant effects of UMB in biochemical assays and cellular reactive oxygen species (ROS) levels in RAW264.7 cells were not significantly improved by addition of other compounds. However, intracellular uptake, inhibition of the efflux pump P-glycoprotein (P-gp), and physiological stability of UMB were greatly enhanced by the addition of other compounds, specifically Angelicin (ANG) and Byakangelicin (BYN). Taken together, enhanced intracellular localization and enzymatic stability in compound mixtures might lead to superior synergistic bioactivity of UMBs in compound mixtures.
TCM-ADMEpred: A novel strategy for poly-pharmacokinetics prediction of traditional Chinese medicine based on single constituent pharmacokinetics, structural similarity, and mathematical modeling.[Pubmed:30826421]
J Ethnopharmacol. 2019 May 23;236:277-287.
ETHNOPHARMACOLOGICAL RELEVANCE: Yuanhu Zhitong prescription (YZP) is a commonly used and relatively simple clinical herb preparation recorded in the China Pharmacopoeia. It contains Corydalis yanhusuo (Chinese name, Yanhusuo [YH]) and Angelica dahurica (Hoffm.) (Chinese name, Baizhi [BZ]), and has a long history of use in traditional Chinese medicine (TCM) for the treatment of stomach pain, hypochondriac pain, headache, and dysmenorrhea. AIM OF THE STUDY: A TCM-ADMEpred method is developed for novel strategy for poly-pharmacokinetics prediction of TCM. To predict the pharmacokinetic characteristics of the main YZP constituents in rat plasma using in silico models, based on the theory that structurally similar constituents show similar pharmacokinetic properties. This approach may facilitate in silico prediction of the pharmacokinetics of TCM. MATERIALS AND METHODS: A robust platform using ultra-performance liquid chromatography coupled with triple quadrupole electrospray tandem mass spectrometry (UPLC-ESI-MS/MS) was developed and validated for simultaneous determination of seven active YZP constituents in rat plasma. These seven compounds were divided into two structural classes, alkaloids and coumarins. The correlation between AUC profiles within a structural class was expressed as Gamma(+), and this variable was used to develop two novel in silico models to predict constituent AUC values. The pharmacokinetics of tetrahydropalmatine, tetrahydroberberine, and corydaline following YZP administration were predicted using the Gamma(+)-values of alpha-allocryptopine observed following YH administration, while those of imperatorin and isoimperatorin following BZ administration were predicted using the Gamma(+)-values of Byakangelicin observed following YZP administration. RESULTS: The UPLC-ESI-MS/MS method was successfully used to evaluate pharmacokinetic parameters after oral YZP, YH, or BZ administration. Our findings showed that co-administration of YH and BZ increased the AUC of four alkaloid constituents and reduced the AUC of three coumarin constituents, which might provide a scientific rationale for co-administering these herbs clinically as a YZP preparation, thus increasing their efficacy and reducing toxicity. The AUC values of imperatorin and isoimperatorin were predicted 3h after oral BZ administration, with the bias ratios between the theoretical values and the observed experimental values ranging from 0.61% to 11.4%, and average bias ratios of 5.8% and 8.0%, respectively. The AUC values of tetrahydropalmatine, tetrahydroberberine, and corydaline were predicted 3h after oral YZP administration, with bias ratios ranging from 3.7% to 46.4%, and average bias ratios of 23.8%, 15.4%, and 25.8%, respectively. CONCLUSION: The UPLC-ESI-MS/MS method was successfully applied to pharmacokinetic evaluations after oral administration of YZP, YH, and BZ to rats. The Gamma(+) variable was used to express the correlation between the AUC profiles of structurally similar compounds. This facilitated the development of an in silico model that was used to predict the AUC of three alkaloids in YZP and of two coumarins in BZ. Calculation of the bias ratios between the predicted and experimental values suggested that this in silico model provided a viable approach for the prediction of TCM pharmacokinetics.
Evaluation of furanocoumarins from seeds of the wild parsnip (Pastinaca sativa L. s.l.).[Pubmed:30562630]
J Chromatogr B Analyt Technol Biomed Life Sci. 2019 Jan 15;1105:54-66.
Although the wild parsnip (Pastinaca sativa L. s.l.) fruits are known to contain linear and angular furanocoumarins, the individual components of the seeds have not been fully identified and quantitated, and, in the case of immature seeds, reported. In view of this, the main furanocoumarin compounds were extracted using pyridine, and were isolated using semi-preparative high-performance liquid chromatography. The structural elucidation of isolated compounds was done based on detailed spectral analysis conducted by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI/MS), (1)H and (13)C NMR and, where possible, by gas chromatography-mass spectrometry (GC-MS). The quantitative analysis of furanocoumarin compounds in the wild parsnip was conducted by analytical ultra-performance liquid chromatography (UPLC-DAD), calculated against the standard curves of isolated compounds. The total yields of furanocoumarin compounds from the seeds after extraction with pyridine were 107.2-222.8mgg(-1) (fresh weight) and 50.2-66.4mgg(-1) (soluble dry matter). Thirteen furanocoumarins were identified. The main compounds (percentage in FW) in immature seeds were bergapten (40.8), pimpinellin (10.5), methoxsalen (5.7), isopimpinellin (4.3), imperatorin (3.2), and phellopterin (7.2). Seven constituents previously not described in P. sativa seeds and its products were identified, namely, byakangelicol (14.4), heraclenin (8.5), isobergapten (2.5), Byakangelicin (1.3), heraclenol (0.5), psoralen (0.3), and isoByakangelicin (0.8). The latter is a new compound of the Apiaceae family. Extraction of immature seeds using pyridine gave a much higher yield and a greater variety of furanocoumarins. This indicates that the wild parsnip, along with other Apiaceae family plants, may be an important source of bioactive compounds.
Separation and determination of coumarins including furanocoumarins using micellar electrokinetic capillary chromatography.[Pubmed:29853023]
Talanta. 2018 Sep 1;187:120-124.
The conditions of micellar electrokinetic capillary chromatography for separation and simultaneous measurement of coumarins (coumarin, scoparone, isoscopoletin, esculin, esculetin, umbelliferone) including furanocoumarins (xanthotoxin, Byakangelicin, isopimpinellin, bergapten, phellopterin, xanthotoxol) have been elaborated. The influence of different parameters, such as the pH of the buffer, sodium cholate (SC) or methanol concentration in the buffer, on the migration time, peak resolution, peak asymmetry, and number of theoretical plates was investigated. The optimum separation of the compounds was achieved using 50-microm i.d. capillaries with a total length of 64.5cm (56cm effective length) and a buffer system at pH 9.00 consisting of 50mM sodium tetraborate, 45mM SC, and 20% of methanol (v/v). The developed method ensured good repeatability of corrected peak areas and migration times (the relative standard deviations were in the range of 2.8-6.1% and 0.8-4.0%, respectively). The average limit of detection for all studied compounds was below 1.3microgmL. Moreover, good linearity of the relationship between the peak corrected area and the concentration of the compounds was observed (correlation coefficient >0.99). The method was successfully applied in the quantitative analysis of two different types of samples, i.e. Heracleum sphondylium herb and Aesculus hippocastanum cortex.
Identification of quality markers of Yuanhu Zhitong tablets based on integrative pharmacology and data mining.[Pubmed:29551644]
Phytomedicine. 2018 May 15;44:212-219.
BACKGROUND: The quality evaluation of traditional Chinese medicine (TCM) formulations is needed to guarantee the safety and efficacy. In our laboratory, we established interaction rules between chemical quality control and biological activity evaluations to study Yuanhu Zhitong tablets (YZTs). Moreover, a quality marker (Q-marker) has recently been proposed as a new concept in the quality control of TCM. However, no appropriate methods are available for the identification of Q-markers from the complex TCM systems. PURPOSE: We aimed to use an integrative pharmacological (IP) approach to further identify Q-markers from YZTs through the integration of multidisciplinary knowledge. In addition, data mining was used to determine the correlation between multiple constituents of this TCM and its bioactivity to improve quality control. METHODS: The IP approach was used to identify the active constituents of YZTs and elucidate the molecular mechanisms by integrating chemical and biosynthetic analyses, drug metabolism, and network pharmacology. Data mining methods including grey relational analysis (GRA) and least squares support vector machine (LS-SVM) regression techniques, were used to establish the correlations among the constituents and efficacy, and dose efficacy in multiple dimensions. RESULTS: Seven constituents (tetrahydropalmatine, alpha-allocryptopine, protopine, corydaline, imperatorin, isoimperatorin, and Byakangelicin) were identified as Q-markers of YZT using IP based on their high abundance, specific presence in the individual herbal constituents and the product, appropriate drug-like properties, and critical contribution to the bioactivity of the mixture of YZT constituents. Moreover, three Q-markers (protopine, alpha-allocryptopine, and corydaline) were highly correlated with the multiple bioactivities of the YZTs, as found using data mining. Finally, three constituents (tetrahydropalmatine, corydaline, and imperatorin) were chosen as minimum combinations that both distinguished the authentic components from false products and indicated the intensity of bioactivity to improve the quality control of YZTs. CONCLUSIONS: Tetrahydropalmatine, imperatorin, and corydaline could be used as minimum combinations to effectively control the quality of YZTs.
[Chemical constituents from lipophilic parts in roots of Angelica dahurica cv.Yubaizhi].[Pubmed:28822155]
Zhongguo Zhong Yao Za Zhi. 2017 Jun;42(11):2102-2109.
The chemical constituents from lipophilic parts in the roots of Angelica dahurica cv. Yubaizhi were studied in this paper. The compounds were separated and purified by repeated column chromatographic methods on silica gel and HPLC, and the chemical structures of compounds were determined by spectral data analyses. Thirty-three compounds were obtained and identified as isoimperatorin (1), imperatorin (2), stigmasterol (3), isooxypeucedanin (4), pabulenol (5), psoralen (6), bergapten (7), isodemethylfuropinarine (8), phellopterin (9), osthenol (10), alloimperatorin (11), xanthotoxin (12), xanthotoxol (13), isopimpinellin (14), alloisoimperatorin (15), beta-sitosterol (16), oxyalloimperatorin (17), pabularinone (18), 5-hydroxy-8-methoxypsoralen (19), columbianetin (20), heracol (21), isogosferol (22), 2''R-neobyakangelicol (23), Byakangelicin ethoxide (24), Byakangelicin (25), oxypeucedanin hydrate (26), uracil (27), umbelliferone (28), bergaptol (29), demethylfuropinarine (30), isobyakangelicol (31), oxypeucedanin ethanolate (32), heraclenol (33). Among them, compounds 8, 10, 17, 21, and 30 were obtained from the roots of title plant for the first time.
Spectrum Effect Relationship and Component Knock-Out in Angelica Dahurica Radix by High Performance Liquid Chromatography-Q Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer.[Pubmed:28754032]
Molecules. 2017 Jul 21;22(7). pii: molecules22071231.
Different extracts of Angelica dahuricae were available for whitening or treating vitiligo clinically. They showed inhibitory or activating effects on tyrosinase, a rate-limiting enzyme of melanogenesis. This study aimed to identify active compounds on tyrosinase in water extract of Angelica dahurica Radix. We applied spectrum-effect relationship and component knock-out methods to make it clear. HPLC was used to obtain the specific chromatograms. The effects on tyrosinase activity were examined by measuring the oxidation rate of levodopa in vitro. Partial least squares method was used to examine the spectrum-effect relationships. The knocked-out samples were prepared by HPLC method, and the identification of knocked-out compounds was conducted by the high performance liquid chromatography-four stage rod-electrostatic field orbit trap high resolution mass spectrometry. Results showed that S6, S14, S18, S21, S35, S36, S37, S40, and S41 were positively correlated to inhibitory activity of Angelica dahuricae on tyrosinase whereas S9, S11, S8, S12, S22, and S30 were negatively correlated. When the concentration of each sample was 1 g.mL(-1), equal to the amount of raw medicinal herbs, oxypeucedanin hydrate, imperatorin, cnidilin, and isoimperatorin had inhibitory effects on tyrosinase activity whereas Byakangelicin and bergapten had activating effects.
Coumarins from the roots of Angelica dahurica cause anti-allergic inflammation.[Pubmed:28673013]
Exp Ther Med. 2017 Jul;14(1):874-880.
Allergic inflammation is induced by allergens and leads to various allergic diseases, including rhinitis, asthma and conjunctivitis. Histamine is important in the pathogenesis of an immunoglobulin E-dependent allergic reaction and results in the secretion of cytokines associated with inflammation. Angelica dahurica (A. dahurica) is a medicinal plant widely used in China for the treatment of symptoms related to allergic inflammation. The present study investigated the chemical constituents from A. dahurica and evaluated their reductive effect on allergic inflammation. As a result, 15 compounds including 13 coumarins have been identified as isoimperatorin (1), imperatorin (2), oxypeucedanin (3), oxypeucedanin hydrate (4), bergapten (5), Byakangelicin (6), phellopterin (7), byakangelicol (8), isopimpinellin (9), xanthotoxol (10), xanthotoxin (11), pimpinellin (12), scopoletin (13), beta-sitosterol (14) and daucosterol (15). Compounds 1-13 were able to reduce the release of histamine, with compounds 4-6 exhibiting the most potent activity. Furthermore, compounds 1-12 were able to inhibit the secretion of tumor necrosis factor-alpha, interleukin (IL)-1beta and IL-4, with compounds 5 and 7 exhibiting the strongest inhibitory effects. These compounds implemented the inhibitory effects on the expression of inflammatory cytokine genes through the inhibition of nuclear factor-kappaB activation. Virtual screening by a docking program indicated that compound 3 is a potent histamine H1 receptor antagonist. Additionally, the calculated physicochemical properties of these compounds support most furanocoumarins to be delivered to binding sites and permeate the cell membrane. The present findings contribute to understanding how A. dahurica attenuates allergic inflammation.


