BoldenoneCAS# 846-48-0 |
Quality Control & MSDS
Number of papers citing our products

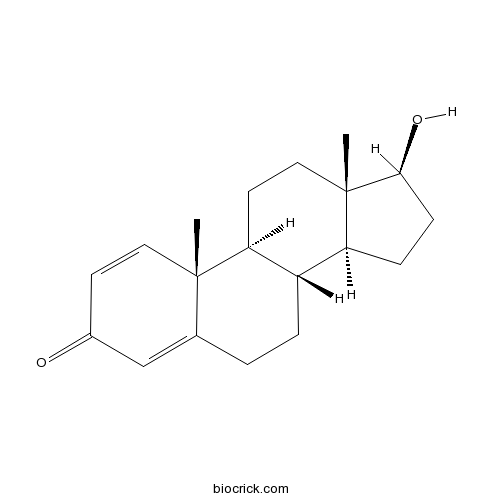
Chemical structure

3D structure
| Cas No. | 846-48-0 | SDF | Download SDF |
| PubChem ID | 13308 | Appearance | Powder |
| Formula | C19H26O2 | M.Wt | 286.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-3-one | ||
| SMILES | CC12CCC3C(C1CCC2O)CCC4=CC(=O)C=CC34C | ||
| Standard InChIKey | RSIHSRDYCUFFLA-DYKIIFRCSA-N | ||
| Standard InChI | InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h7,9,11,14-17,21H,3-6,8,10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boldenone Dilution Calculator

Boldenone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4916 mL | 17.4581 mL | 34.9162 mL | 69.8324 mL | 87.2905 mL |
| 5 mM | 0.6983 mL | 3.4916 mL | 6.9832 mL | 13.9665 mL | 17.4581 mL |
| 10 mM | 0.3492 mL | 1.7458 mL | 3.4916 mL | 6.9832 mL | 8.7291 mL |
| 50 mM | 0.0698 mL | 0.3492 mL | 0.6983 mL | 1.3966 mL | 1.7458 mL |
| 100 mM | 0.0349 mL | 0.1746 mL | 0.3492 mL | 0.6983 mL | 0.8729 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5α-Androstanedione
Catalog No.:BCC8752
CAS No.:846-46-8
- Serrin A
Catalog No.:BCN6985
CAS No.:845959-98-0
- 2-Methyl-5-hydroxytryptamine hydrochloride
Catalog No.:BCC5663
CAS No.:845861-49-6
- CP 94253 hydrochloride
Catalog No.:BCC7018
CAS No.:845861-39-4
- Bakuchalcone
Catalog No.:BCN3201
CAS No.:84575-13-3
- Cleomiscosin C
Catalog No.:BCN4388
CAS No.:84575-10-0
- Rocaglamide
Catalog No.:BCN4387
CAS No.:84573-16-0
- PHA-767491
Catalog No.:BCC1858
CAS No.:845714-00-3
- 4-Hydroxycephalotaxine
Catalog No.:BCN4386
CAS No.:84567-08-8
- Bitopertin (R enantiomer)
Catalog No.:BCC1420
CAS No.:845614-12-2
- Bitopertin
Catalog No.:BCC1419
CAS No.:845614-11-1
- MNI-caged-D-aspartate
Catalog No.:BCC5896
CAS No.:845555-94-4
- Lorazepam
Catalog No.:BCC5970
CAS No.:846-49-1
- Cyclogalegigenin
Catalog No.:BCN6295
CAS No.:84605-18-5
- Fmoc-D-Val-OH
Catalog No.:BCC3573
CAS No.:84624-17-9
- Boc-Lys(Fmoc)-OH
Catalog No.:BCC3417
CAS No.:84624-27-1
- H-Lys(Fmoc)-OH
Catalog No.:BCC2984
CAS No.:84624-28-2
- Itraconazole
Catalog No.:BCC4914
CAS No.:84625-61-6
- Eurycomanone
Catalog No.:BCN2990
CAS No.:84633-29-4
- 4-Nitrobenzyl dimethylcarbamate
Catalog No.:BCN3284
CAS No.:84640-31-3
- Tea polyphenol
Catalog No.:BCN8518
CAS No.:84650-60-2
- Decinnamoyltaxinine J
Catalog No.:BCN7210
CAS No.:84652-33-5
- Lorcaserin HCl
Catalog No.:BCC5041
CAS No.:846589-98-8
- Isoastragaloside I
Catalog No.:BCN2979
CAS No.:84676-88-0
Elucidation of the biosynthetic pathways of boldenone in the equine testis.[Pubmed:30951760]
Steroids. 2019 Apr 2;146:79-91.
Boldenone is an anabolic-androgenic steroid that is prohibited in equine sports. Urine from the uncastrated male horse contains Boldenone that is thought to be of endogenous origin and thus a threshold ('cut-off') concentration has been adopted internationally for free and conjugated Boldenone to help distinguish cases of doping from its natural production. The testis is likely to be a source of Boldenone. Qualitative analysis was performed on extracts of equine testicular homogenates (n=3 horses) incubated non-spiked and in the presence of its potential precursors using liquid chromatography tandem mass spectrometry (LC-MS/MS) and LC high resolution mass spectrometry (LC-HRMS). Samples were analysed both underivatised and derivatised to increase the certainty of identification. In addition to previously reported endogenous steroids, analysis of non-spiked testicular tissue samples demonstrated the presence of Boldenone and boldienone at trace levels in the equine testis. Incubation of homogenates with deuterium or carbon isotope labelled testosterone and androstenedione resulted in the matching stable isotope analogues of Boldenone and boldienone being formed. Additionally, deuterium and carbon labelled 2-hydroxyandrostenedione was detected, raising the possibility that this steroid is a biosynthetic intermediate. In conclusion, Boldenone and boldienone are naturally present in the equine testis, with the biosynthesis of these steroids arising from the conversion of testosterone and androstenedione. However, additional work employing larger numbers of animals, further enzyme kinetic experiments and pure reference standards for 2-OH androstenedione isomers would be required to better characterize the pathways involved in these transformations.
Determination of boldenone in postmortem specimens including blood and urine samples using LC-MS/MS.[Pubmed:30851513]
J Pharm Biomed Anal. 2019 May 30;169:111-115.
Boldenone (BOLD), one of androgenic anabolic steroids (AAS), although banned in humans, is still available illegally. AAS abuse has previously been associated with various cardiovascular adverse events including acute myocardial infarction, arrhythmia, and sudden death. In this study, the concentration of BOLD was determined in postmortem specimens from the corpse of a human male who intentionally injected BOLD undecylenate into his shoulder muscle. In addition, the endogenous levels of BOLD in the blood and urine samples of young human males have been reported. A liquid chromatography-tandem mass spectrometry (LC-MS/MS) method with solid-phase extraction (SPE) was developed and validated for the analysis of BOLD in blood, muscular tissue and urine samples. The validation parameters including linearity, accuracy, precision, matrix effect, and recovery were satisfactory. The concentrations of BOLD in the blood of 20 young human males who didn't take BOLD were under the limit of quantitation (LOQ, 0.5 ng/mL). Additionally, the mean level of BOLD in the urine samples was 3.19 +/- 1.65 ng/mL (range: 0.37 6.02 ng/mL). The concentrations of BOLD in the victim's blood from the femoral vein and heart were 140.44 and 25.74 ng/mL, respectively. On the other hand, those in the muscular tissue from the injection site and the urine sample were 142.3 ng/g and 3474 ng/mL, respectively.
Highly efficient synthesis of boldenone from androst-4-ene-3,17-dione by Arthrobacter simplex and Pichia pastoris ordered biotransformation.[Pubmed:30848360]
Bioprocess Biosyst Eng. 2019 Mar 8. pii: 10.1007/s00449-019-02092-y.
Boldenone (BD) is an important steroid hormone drug which is the derivative of testosterone. In this study, an ordered biotransformation method was proposed employing Arthrobacter simplex and recombinant Pichia pastoris with 17beta-hydroxysteroid dehydrogenase from Saccharomyces cerevisiae to produce BD from androst-4-ene-3,17-dione (AD) efficiently. To lower the oxidation towards BD in A. simplex, the transformation was conducted sequentially by C1,2 dehydrogenation in A. simplex and 17beta-carbonyl reduction in recombinant P. pastoris GS115. Moreover, the reaction system was inactivated before recombinant P. pastoris GS115 was added to further inhibit the oxidation of BD by A. simplex, and the productivity of BD was improved 10.6% compared with the control. Furthermore, by optimizing the conditions of transformation from AD to BD, 4.2 g/L BD was generated with 83% productivity from 5.0 g/L AD, which was the highest productivity reported by biological method. This study offers a promising method to produce BD by ordered biotransformation system, which can also be used to manufacture other steroidal compounds that are difficult to acquire directly.
Phytochemical profile, anti-oxidant, anti-inflammatory, and anti-proliferative activities of Pogostemon deccanensis essential oils.[Pubmed:30622869]
3 Biotech. 2019 Jan;9(1):31.
Essential oils (EOs) obtained from aerial parts of Pogostemon deccanensis were analyzed for GC-MS profiling, and evaluated for antioxidant, anti-inflammatory, and anti-proliferative activities. GC-MS analysis revealed a total of 47 constituents, establishing the EOs rich in sesquiterpene with > 20 sesquiterpenes constituting around 77% of the total EO yield. Major constituents included Curzerene (Benzofuran, 6-ethenyl-4,5,6,7-tetrahydro-3,6-dimethyl-5-isopropenyl-, trans-) (26.39%) and epi-Cadinol (22.68%), Ethanone, 1-(2,4,6-trihydroxyphenyl) (6.83%, Acetophenones), and Boldenone (3.47%, anabolic steroid). EOs found to be rich in phytochemicals attributed for antioxidant potentials of aromatic/medicinal plants, viz., flavonoids (2.71 microg quercetin equivalents g(-1) EO), total phenols (3.94 microg gallic acid equivalents (GAE) g(-1) EO), carotenoids (14.3 microg beta-carotene equivalents g(-1) EO), and ascorbic acid (2.21 microg ascorbic acid equivalents g(-1) EO). P. deccanensis EOs exhibited striking antioxidant activities assessed by wide range of assays including ferric reducing antioxidant potential (FRAP, 255.3 GAE at 2 microg mL(-1) EO), total antioxidant activity (TAA, 264.3 GAE at 2 microg ml(-1)) of EO, DPPH (65% inhibition at 2 microg mL(-1)), and OH (58% inhibition at 2 microg mL(-1)) scavenging. Interestingly, EOs showed considerably higher anti-lipid peroxidation activity than the standard antioxidant molecule ascorbic acid, with 50% protection by 1.29 microg mL(-1) EO against 20.0 microg mL(-1) standard. EOs showed strong anti-inflammatory activity with 50% inhibition at 1.95 microg mL(-1) EO. The anti-proliferative activity of EOs was tested against mouse cancer cell line and the EOs proved a potent anti-proliferative agent with only 2.1% cell survival at 2 microg mL(-1) EO, whereas the EOs were largely non-toxic-to-normal (non-cancerous) cells with approximately 80% cell survival at the 2 microg mL(-1) EOs. This being the first attempt of phytochemical profiling and wide array of biological activities of P. deccanensis EOs holds significance as the striking activities were observed at very low concentrations, in some cases at lower than the commercial standards, and has, therefore, great potential for pharmaceutical or commercial exploration.
Grape Seed Proanthocyanidin Ameliorates Cardiac Toxicity Induced by Boldenone Undecylenate through Inhibition of NADPH Oxidase and Reduction in the Expression of NOX2 and NOX4.[Pubmed:30116498]
Oxid Med Cell Longev. 2018 Jul 5;2018:9434385.
The effect of anabolic androgenic steroids on the cardiovascular system is poorly understood. Increased production of free radicals is coupled to the pathophysiology of many alterations within the circulatory system. The only function of the enzyme family NADPH oxidases (NOXs) is the generation of reactive oxygen species (ROS). Therefore, this study investigated the beneficial role of grape seed proanthocyanidin extract (GSPE) in ameliorating cardiac toxicity induced by the anabolic steroid Boldenone in male rats through NOX inhibition and reduction in the expression of NOX2 and NOX4. This study was conducted on forty male rats which are divided into four groups (normal control, positive control or GSPE, Boldenone, and posttreatment Boldenone with GSPE). A significant increase in relative body weight, relative heart weight, and hemodynamic parameters, as well as serum concentrations of lactate dehydrogenase, creatine kinase, creatine kinase-muscle brain, myoglobin, cholesterol, low-density lipoprotein cholesterol, risk factor 1/2, K(+), and Cl(-), in treated rats with Boldenone when compared with control. We also noted a significant increase in the levels of cardiac malondialdehyde, H2O2 generation in heart tissues, mRNA expression of NOX2 and NOX4, and immunoreactivity to proliferating cell nuclear antigen (PCNA). Posttreated rats with Boldenone and GSPE ameliorated cardiac toxicity via inhibition of NOX and a reduction in alteration of the expression of NOX2, NOX4, and PCNA induced by Boldenone. These novel insights into the antioxidative activity of GSPE should serve as a basis for the development of improved chemopreventive or therapeutic strategies for cardiac toxicity.


