BL 1249Putative activator of potassium TREK-1 CAS# 18200-13-0 |
- A66
Catalog No.:BCC3715
CAS No.:1166227-08-2
- PI3k-delta inhibitor 1
Catalog No.:BCC1861
CAS No.:1332075-63-4
- PIK-75
Catalog No.:BCC1163
CAS No.:372196-77-5
- CAL-101 (Idelalisib, GS-1101)
Catalog No.:BCC1270
CAS No.:870281-82-6
- PIK-294
Catalog No.:BCC4995
CAS No.:900185-02-6
- PI-3065
Catalog No.:BCC5379
CAS No.:955977-50-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18200-13-0 | SDF | Download SDF |
| PubChem ID | 16078951 | Appearance | Powder |
| Formula | C17H17N5 | M.Wt | 291.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. NaOH and to 100 mM in DMSO | ||
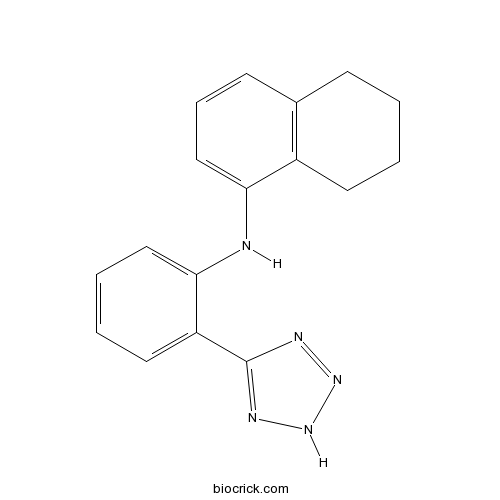
| Chemical Name | N-[2-(2H-tetrazol-5-yl)phenyl]-5,6,7,8-tetrahydronaphthalen-1-amine | ||
| SMILES | C1CCC2=C(C1)C=CC=C2NC3=CC=CC=C3C4=NNN=N4 | ||
| Standard InChIKey | YYNRZIFBTOUICE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H17N5/c1-2-8-13-12(6-1)7-5-11-15(13)18-16-10-4-3-9-14(16)17-19-21-22-20-17/h3-5,7,9-11,18H,1-2,6,8H2,(H,19,20,21,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Putative potassium channel activator; thought to act as a K2P2.1 (TREK-1) channel opener. Exhibits selectivity for bladder over vascular tissue in vitro and in vivo (EC50 values are 1.26 and 21.0 μM for cultured bladder and aortic tissues respectively). |

BL 1249 Dilution Calculator

BL 1249 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4323 mL | 17.1615 mL | 34.323 mL | 68.646 mL | 85.8074 mL |
| 5 mM | 0.6865 mL | 3.4323 mL | 6.8646 mL | 13.7292 mL | 17.1615 mL |
| 10 mM | 0.3432 mL | 1.7161 mL | 3.4323 mL | 6.8646 mL | 8.5807 mL |
| 50 mM | 0.0686 mL | 0.3432 mL | 0.6865 mL | 1.3729 mL | 1.7161 mL |
| 100 mM | 0.0343 mL | 0.1716 mL | 0.3432 mL | 0.6865 mL | 0.8581 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
[(5, 6, 7, 8-tetrahydro-naphthalen-1-yl)-[2-(1H-tetrazol-5-yl)-phenyl]-amine], named BL 1249, is a putative potassium channel opener with bladder-relaxant properties. In cultured bladder smooth muscle cells, BL 1249 decreased DiBAC4 (3) fluorescence in a concentration-dependent manner with an EC50 of 1.26 ± 0.6 µM. In human bladder cells, BL 1249 resulted in hyperpolarization in a concentration-dependent manner and yielded an EC50 of 1.49±0.08 µM. BL 1249 relaxed 30 mM KCl precontracted bladder strips in a concentration-dependent manner and yielded an EC50 of 1.1 ± 0.37 µM [1].
There are several types of potassium channels in urinary bladder myocytes and they are important in determining contractility and excitability of bladder smooth muscle. These channels include maxi-K+, KATP, members of the voltage-gated Kv family, SK family and, possibly, members of the KCNQ family. Open potassium channels can increase potassium efflux from the cell and produce membrane potential hyperpolarization, thereby decrease the activation of voltage-dependent calcium channels [1].
In human bladder myocytes, BL 1249 produced large instantaneously non-inactivating, activating outward currents that were readily reversible following drug washout. The BL 1249-induced current was hence of a reversal potential of near -80 mV under the physiological K+ gradient, this indicated that the current is carried by K+ ions [1].
In anesthetized rats, BL 1249 at a concentration of 1 mg/kg significantly decreased (p< 0.01) micturition contractions during the 15-min period immediately following dosing; for the 15- to 30-min period, the decrease was significant but less (p< 0.05). Administration of BL 1249 at a concentration of 1 mg/kg had no effect on mean arterial blood pressure (MABP) during the 0- to 15-min period immediately following administration. During the 15- to 30-min period, BL 1249 administration increased MABP by less than 10% [1].
Reference:
[1]. Svetlana Tertyshnikova, Ronald J. Knox, Mary Jane Plym, et al. BL-1249 [(5, 6, 7, 8-Tetrahydro-naphthalen-1-yl)-[2-(1H-tetrazol-5-yl)-phenyl]-amine]: A Putative Potassium Channel Opener with Bladder-Relaxant Properties. The Journal of Pharmacology and Experimental Therapeutics, 2015, 313(1):250-259.
- Ethyl beta-D-fructofuranoside
Catalog No.:BCN1145
CAS No.:1820-84-4
- Angenomalin
Catalog No.:BCN8246
CAS No.:18199-64-9
- Naringenin-4',7-diacetate
Catalog No.:BCN1144
CAS No.:18196-13-9
- Fmoc-Dap-OH
Catalog No.:BCC3187
CAS No.:181954-34-7
- Sequirin C
Catalog No.:BCN4688
CAS No.:18194-29-1
- DLPC
Catalog No.:BCC7929
CAS No.:18194-25-7
- KC 12291 hydrochloride
Catalog No.:BCC7618
CAS No.:181936-98-1
- Crotonoside
Catalog No.:BCN6281
CAS No.:1818-71-9
- 25-Anhydrocimigenol 3-O-beta-D-xyloside
Catalog No.:BCN3436
CAS No.:181765-11-7
- (-)-beta-Pinene
Catalog No.:BCN3857
CAS No.:18172-67-3
- Cyclopiazonic acid
Catalog No.:BCC6981
CAS No.:18172-33-3
- Interiotherins A
Catalog No.:BCN3093
CAS No.:181701-06-4
- Meliasendanin D
Catalog No.:BCN7610
CAS No.:1820034-05-6
- KB-R7943 mesylate
Catalog No.:BCC1676
CAS No.:182004-65-5
- 6-Angeloyloxyditropan-3-yl itaconate
Catalog No.:BCN1867
CAS No.:182015-05-0
- Nitrosostromelin
Catalog No.:BCN1745
CAS No.:182064-61-5
- Quinovic acid 3-O-(3',4'-O-isopropylidene)-beta-D-fucopyranoside
Catalog No.:BCN1519
CAS No.:182132-59-8
- Nyssoside
Catalog No.:BCN1146
CAS No.:182138-70-1
- 3,4-seco-Olean-12-en-4-ol-3,28-dioic acid
Catalog No.:BCN7151
CAS No.:182249-69-0
- Clausine I
Catalog No.:BCN4687
CAS No.:182261-94-5
- Lirioprolioside B
Catalog No.:BCN2740
CAS No.:182284-68-0
- Synthalin sulfate
Catalog No.:BCC6730
CAS No.:182285-12-7
- Antibiotic ZG 1494alpha
Catalog No.:BCN1850
CAS No.:182320-33-8
- Antibiotic 2158
Catalog No.:BCN1825
CAS No.:182320-34-9
BL-1249 [(5,6,7,8-tetrahydro-naphthalen-1-yl)-[2-(1H-tetrazol-5-yl)-phenyl]-amine]: a putative potassium channel opener with bladder-relaxant properties.[Pubmed:15608074]
J Pharmacol Exp Ther. 2005 Apr;313(1):250-9.
BL-1249 [(5,6,7,8-tetrahydro-naphthalen-1-yl)-[2-(1H-tetrazol-5-yl)-phenyl]-amine] produced a concentration-dependent membrane hyperpolarization of cultured human bladder myocytes, assessed as either a reduction in fluorescence of the voltage-sensitive dye bis-(1,2-dibutylbarbituric acid)trimethine oxonol (EC50 = 1.26 +/- 0.6 microM) or by direct electrophysiological measurement (EC50 = 1.49 +/- 0.08 microM). BL-1249 also produced a membrane hyperpolarization of acutely dissociated rat bladder myocytes. Voltage-clamp studies in human bladder cells revealed that BL-1249 activated an instantaneous, noninactivating current that reversed near E(K). The BL-1249-evoked outward K+ current was insensitive to blockade by glyburide, tetraethylammonium, iberiotoxin, 4-aminopyridine, apamin, or Mg2+. However, the current was inhibited by extracellular Ba2+ (10 mM). In in vitro organ bath experiments, BL-1249 produced a concentration-dependent relaxation of 30 mM KCl-induced contractions in rat bladder strips (EC50 = 1.12 +/- 0.37 microM), yet had no effect on aortic strips up to the highest concentration tested (10 microM). The bladder relaxation produced by BL-1249 was partially blocked by Ba2+ (1 and 10 mM) but not by apamin, iberiotoxin, 4-aminopyridine, glyburide, or tetraethylammonium. In an anesthetized rat model, BL-1249 (1 mg/kg i.v.) decreased the number of isovolumic contractions, without significantly affecting blood pressure. Thus, BL-1249 behaves as a potassium channel activator that exhibits bladder versus vascular selectivity both in vitro and in vivo. A survey of potassium channels exhibiting sensitivity to extracellular Ba2+ at millimolar concentration revealed that the expression of the K2P2.1 (TREK-1) channel was relatively high in human bladder cells versus human aortic cells, suggesting this channel as a possible candidate target for BL-1249.
Investigation of the role of TASK-2 channels in rat pulmonary arteries; pharmacological and functional studies following RNA interference procedures.[Pubmed:16432512]
Br J Pharmacol. 2006 Mar;147(5):496-505.
In the present study, we investigated the ability of RNA interference technology to suppress TASK-2 potassium channel expression in human embryonic kidney (HEK293) cells stably transfected with TASK-2 cDNA and in rat isolated intact pulmonary arteries. Lipofectamine-induced transfection of a specific siRNA sequence targeted against TASK-2 resulted in a dose- and time-dependent decrease in TASK-2 channel protein expression. In siRNA-transfected cells the TASK-2 peak currents were significantly smaller than in control cells at every investigated pH, while the pH sensitivity was not altered. Using scrambled siRNA as a negative control, there were no significant changes in TASK-2 protein expression or current compared to mock-transfected cells. In TASK-2 siRNA-transfected small pulmonary arteries, but not in scrambled siRNA-treated vessels, myocyte resting membrane potential at pH 7.4 was significantly less negative and the hyperpolarisations in response to increasing pH from 6.4 to 8.4 were significantly smaller compared with control. The application of levcromakalim (10 microM), NS1619 (33 microM) and a potassium channel inhibitor cocktail (5 mM 4-aminopyridine, 10 mM tetraethylammonium chloride, 30 microM Ba2+ and 10 microM glibenclamide) had similar effects in control and in siRNA-transfected vessels. The TASK-1 (anandamide-sensitive) contribution to resting membrane potential was comparable in each group. Clofilium (100 microM) generated significantly smaller responses in transfected artery segments. These results suggest that RNA interference techniques are effective at inhibiting TASK-2 channel expression in cultured cells and in intact vessels and that TASK-2 channels have a functional role in setting the membrane potential of pulmonary artery myocytes.


