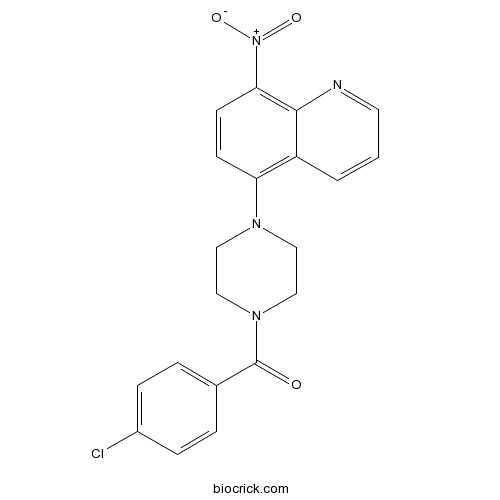
B2Promotes inclusion formation in Huntington's and Parkinson's diseases CAS# 115687-05-3 |
- CEP-32496
Catalog No.:BCC1079
CAS No.:1188910-76-0
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- BRAF inhibitor
Catalog No.:BCC1436
CAS No.:918505-61-0
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115687-05-3 | SDF | Download SDF |
| PubChem ID | 3953303 | Appearance | Powder |
| Formula | C20H17ClN4O3 | M.Wt | 396.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CPNQ | ||
| Solubility | Soluble to 100 mM in DMSO and to 10 mM in ethanol | ||
| Chemical Name | (4-chlorophenyl)-[4-(8-nitroquinolin-5-yl)piperazin-1-yl]methanone | ||
| SMILES | C1CN(CCN1C2=C3C=CC=NC3=C(C=C2)[N+](=O)[O-])C(=O)C4=CC=C(C=C4)Cl | ||
| Standard InChIKey | KLNPVNZJCWIQSK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H17ClN4O3/c21-15-5-3-14(4-6-15)20(26)24-12-10-23(11-13-24)17-7-8-18(25(27)28)19-16(17)2-1-9-22-19/h1-9H,10-13H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Promotes inclusion formation in cellular models of Huntington's disease and Parkinson's disease. Prevents mutant huntingtin-mediated proteasome dysfunction and reduces α-synuclein-mediated toxicity. |

B2 Dilution Calculator

B2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.52 mL | 12.5999 mL | 25.1997 mL | 50.3994 mL | 62.9993 mL |
| 5 mM | 0.504 mL | 2.52 mL | 5.0399 mL | 10.0799 mL | 12.5999 mL |
| 10 mM | 0.252 mL | 1.26 mL | 2.52 mL | 5.0399 mL | 6.2999 mL |
| 50 mM | 0.0504 mL | 0.252 mL | 0.504 mL | 1.008 mL | 1.26 mL |
| 100 mM | 0.0252 mL | 0.126 mL | 0.252 mL | 0.504 mL | 0.63 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-DL-Pro-NH2
Catalog No.:BCC3027
CAS No.:115630-49-4
- Cinnamyl caffeate
Catalog No.:BCN7721
CAS No.:115610-32-7
- Cinnamyl isoferulate
Catalog No.:BCN7718
CAS No.:115610-31-6
- Cinnamyl coumarate
Catalog No.:BCN7739
CAS No.:115610-30-5
- 4-Androstenediol
Catalog No.:BCC8692
CAS No.:1156-92-9
- 2'-Hydroxygenistein
Catalog No.:BCN6036
CAS No.:1156-78-1
- Marbofloxacin hydrochloride
Catalog No.:BCC4250
CAS No.:115551-26-3
- Marbofloxacin
Catalog No.:BCC2510
CAS No.:115550-35-1
- BI 224436
Catalog No.:BCC5531
CAS No.:1155419-89-8
- Fmoc-Cys(Trt)-Opfp
Catalog No.:BCC3480
CAS No.:115520-21-3
- (2-Amino-1-hydroxyethyl)phosphonic acid
Catalog No.:BCN1613
CAS No.:115511-00-7
- H-Lys(Z)-OH
Catalog No.:BCC2986
CAS No.:1155-64-2
- Daidzein dimethyl ether
Catalog No.:BCN6761
CAS No.:1157-39-7
- Adonifoline
Catalog No.:BCN2056
CAS No.:115712-88-4
- 6'-O-beta-D-Glucosylgentiopicroside
Catalog No.:BCN2814
CAS No.:115713-06-9
- 1-O-galloyl-6-O-cinnamoylglucose
Catalog No.:BCN8264
CAS No.:115746-69-5
- Galanolactone
Catalog No.:BCN6037
CAS No.:115753-79-2
- 15,16-Dihydro-15-methoxy-16-oxohardwickiic acid
Catalog No.:BCN1612
CAS No.:115783-35-2
- ent-Atisane-3beta,16alpha,17-triol
Catalog No.:BCN6626
CAS No.:115783-44-3
- Tubeimoside II
Catalog No.:BCN2955
CAS No.:115810-12-3
- Tubeimoside III
Catalog No.:BCN2956
CAS No.:115810-13-4
- 2,7-Dideacetoxytaxinine J
Catalog No.:BCN7281
CAS No.:115810-14-5
- Salvianolic acid C
Catalog No.:BCN5376
CAS No.:115841-09-3
- Aurora A Inhibitor I
Catalog No.:BCC2182
CAS No.:1158838-45-9
Intramembranous processing by gamma-secretase regulates reverse signaling of ephrin-B2 in migration of microglia.[Pubmed:28370426]
Glia. 2017 Jul;65(7):1103-1118.
The Eph-ephrin system plays pivotal roles in cell adhesion and migration. The receptor-like functions of the ephrin ligands allow the regulation of intracellular processes via reverse signaling. gamma-Secretase mediated processing of ephrin-B has previously been linked to activation of Src, a kinase crucial for focal adhesion and podosome phosphorylation. Here, we analyzed the role of gamma-secretase in the stimulation of reverse ephrin-B2 signaling in the migration of mouse embryonic stem cell derived microglia. The proteolytic generation of the ephrin-B2 intracellular domain (ICD) by gamma-secretase stimulates Src and focal adhesion kinase (FAK). Inhibition of gamma-secretase decreased the phosphorylation of Src and FAK, and reduced cell motility. These effects were associated with enlargement of the podosomal surface. Interestingly, expression of ephrin-B2 ICD could rescue these effects, indicating that this proteolytic fragment mediates the activation of Src and FAK, and thereby regulates podosomal dynamics in microglial cells. Together, these results identify gamma-secretase as well as ephrin-B2 as regulators of microglial migration.
Bradykinin/B2 receptor activation regulates renin in M-1 cells via protein kinase C and nitric oxide.[Pubmed:28373410]
Physiol Rep. 2017 Apr;5(7). pii: 5/7/e13211.
In the collecting duct (CD), the interactions of renin angiotensin system (RAS) and kallikrein-kinin system (KKS) modulate Na(+) reabsorption, volume homeostasis, and blood pressure. In this study, we used a mouse kidney cortical CD cell line (M-1 cells) to test the hypothesis that in the CD, the activation of bradykinin B2 receptor (B2R) increases renin synthesis and release. Physiological concentrations of bradykinin (BK) treatment of M-1 cells increased renin mRNA and prorenin and renin protein contents in a dose-dependent manner and increased threefold renin content in the cell culture media. These effects were mediated by protein kinase C (PKC) independently of protein kinase A (PKA) because B2R antagonism with Icatibant and PKC inhibition with calphostin C, prevented these responses, but PKA inhibition with H89 did not modify the effects elicited by the B2R activation. BK-dependent stimulation of renin gene expression in CD cells also involved nitric oxide (NO) pathway because increased cGMP levels and inhibition of NO synthase with L-NAME prevented it. Complementary renin immunohistochemical studies performed in kidneys from mice with conventional B2R knockout and conditional B2R knockout in the CD, showed marked decreased renin immunoreactivity in CD, regardless of the renin presence in juxtaglomerular cells in the knockout mice. These results indicate that the activation of B2R increases renin synthesis and release by the CD cells through PKC stimulation and NO release, which support further the interactions between the RAS and KKS.
Infrared Multiple-Photon Dissociation Action Spectroscopy of the b2(+) Ion from PPG: Evidence of Third Residue Affecting b2(+) Fragment Structure.[Pubmed:28374317]
J Am Soc Mass Spectrom. 2017 Jul;28(7):1482-1488.
Infrared multiple-photon dissociation (IRMPD) action spectroscopy was performed on the B2(+) fragment ion from the protonated PPG tripeptide. Comparison of the experimental infrared spectrum with computed spectra for both oxazolone and diketopiperazine structures indicates that the majority of the fragment ion population has an oxazolone structure with the remainder having a diketopiperazine structure. This result is in contrast with a recent study of the IRMPD action spectrum of the PP B2(+) fragment ion from PPP, which was found to be nearly 100% diketopiperazine (Martens et al. Int. J. Mass Spectrom. 2015, 377, 179). The diketopiperazine B2(+) ion is thermodynamically more stable than the oxazolone but normally requires a trans/cis peptide bond isomerization in the dissociating peptide. Martens et al. showed through IRMPD action spectroscopy that the PPP precursor ion was in a conformation in which the first peptide bond is already in the cis conformation and thus it was energetically favorable to form the thermodynamically-favored diketopiperazine B2(+) ion. In the present case, solution-phase NMR spectroscopy and gas-phase IRMPD action spectroscopy show that the PPG precursor ion has its first amide bond in a trans configuration suggesting that the third residue is playing an important role in both the structure of the peptide and the associated ring-closure barriers for oxazolone and diketopiperazine formation. Graphical Abstract .
Spread of CTX-Type Extended-Spectrum beta-Lactamase-Producing Escherichia coli Isolates of Epidemic Clone B2-O25-ST131 Among Dogs and Cats in Japan.[Pubmed:28380311]
Microb Drug Resist. 2017 Dec;23(8):1059-1066.
This study was performed to investigate the carriage rates of CTX-M-type extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli among ill companion animals in Japan. Among the 178 nonrepetitive E. coli isolates, including 131 from dogs and 47 from cats, collected between September and November 2015, 42 (23.6%) isolates from 29 dogs and 13 cats were identified as ESBL producers. The antimicrobial susceptibility, O serotype, phylogenetic group, beta-lactamase genotype, plasmid replicon type, and sequence type (ST) of each isolate were analyzed. The major ESBL types were CTX-M-14 (26.8%), CTX-M-15 (24.4%), CTX-M-27 (19.5%), and CTX-M-55 (19.5%); predominant replicon types of blaCTX-M-carrying plasmid were IncF group and IncI1-Igamma. The most prevalent STs were ST131 (n = 15, 35.7%), followed by ST38, ST10, and ST410. The 15 isolates of ST131 belonged to B2-O25. E. coli B2-O25-ST131 isolates harboring blaCTX-M-15 or blaCTX-M-27 were resistant to ceftazidime and ciprofloxacin. In particular, CTX-M-15 producers showed multidrug resistance. Our results demonstrated that the CTX-M-producing pandemic E. coli clone B2-O25-ST131 has already spread in Japanese companion animals as well. Moreover, the similarity of genotypes, serotypes, phylogenetic groups, and STs of the isolates from companion animals to those from humans suggested probable transmission of resistant bacteria between pets and humans.


